WebThe solubility in common organic solvents makes it easier to process high-quality films for optoelectronic devices. We normally draw an "exploded" version which looks like this: Only those ions joined by lines are actually touching each other. But with NaCl, the sodium ion is only about 52% of the size of the chloride ion. Greatly suppressed nonradiative decay is achieved for the 2-DC structures. The attractions between the solvent molecules and the ions are not big enough to overcome the attractions holding the crystal together. The napthelenide salt seems a bit too scary for this (we have certain conditions on what is in the detector cell because of where we want to put it) same for the perchlorate. M Logitech Co., Ltd. Cu 2+ + 2I <> Hu, Z.; Huang, G.; Lustig, W.P. There could be billions of sodium ions and chloride ions packed together, or trillions, or whatever - it simply depends how big the crystal is. All compounds are two-dimensional (2D) networks made of one-dimensional (1D) copper iodide staircase chains that are interconnected by bidentate nitrogen-containing ligands. Polyhalide-bonded metal complexes: Structural diversity in an eclectic class of compounds.  139, Issue 27, Applied Physics Letters, Vol. 12 Soluble glacial acetic acid; relatively insol dichloromethane. Organic-based electrolytes, such as methanol, acetonitrile, and dimethylformamide, have been explored as an The greatly enhanced solubility is a result of incorporation of ionic bonds into extended covalent/coordinate network structures, making it possible to fabricate large scale thin films by solution processes. Solubility of lithium salts in organic solvents Biol Psychiatry. 142, Issue 9, Chemical Society Reviews, Vol. Here, using ; Adonin, S.A. Heteroleptic Cu(I) halide complexes with perchlorinated 1,10-phenanthroline. We'll look first at the arrangement of the ions and then talk about why the structures of sodium chloride and cesium chloride are different afterwards. WebIn the laboratory, copper(I) Iodide is prepared by simply mixing an aqueous solutions of sodium or potassium iodide and a soluble copper(II) salt such copper sulfate. ; Dey, G.; Shen, Z.; Wang, L.; OCarroll, D.M. WebCopper(I) chloride (quite commonly called cuprous chloride), is the lower chloride of copper, with the formula CuCl.It occurs naturally as the mineral nantokite. MathJax reference. A New Internet Generation is coming, and we aim to be a part of it inspiring, creating products under the philosophy that the users have control of their data and democratizing the Internet through a process of decentralization. WebThe solubility of Copper iodide (CuI) in water and dimethyl formamide (DMF) with potassium iodide in the combination as KI + Water, and KI + DMF were measured using an analytical gravimetric method at temperatures ranging from (298.15 to 315.15) K. The densities of the saturated solutions for both combinations are also reported. <> Bi, M.; Li, G.; Zou, Y.; Shi, Z.; Feng, S. Zeolite-like Copper Iodide Framework with New 66 Topology.
139, Issue 27, Applied Physics Letters, Vol. 12 Soluble glacial acetic acid; relatively insol dichloromethane. Organic-based electrolytes, such as methanol, acetonitrile, and dimethylformamide, have been explored as an The greatly enhanced solubility is a result of incorporation of ionic bonds into extended covalent/coordinate network structures, making it possible to fabricate large scale thin films by solution processes. Solubility of lithium salts in organic solvents Biol Psychiatry. 142, Issue 9, Chemical Society Reviews, Vol. Here, using ; Adonin, S.A. Heteroleptic Cu(I) halide complexes with perchlorinated 1,10-phenanthroline. We'll look first at the arrangement of the ions and then talk about why the structures of sodium chloride and cesium chloride are different afterwards. WebIn the laboratory, copper(I) Iodide is prepared by simply mixing an aqueous solutions of sodium or potassium iodide and a soluble copper(II) salt such copper sulfate. ; Dey, G.; Shen, Z.; Wang, L.; OCarroll, D.M. WebCopper(I) chloride (quite commonly called cuprous chloride), is the lower chloride of copper, with the formula CuCl.It occurs naturally as the mineral nantokite. MathJax reference. A New Internet Generation is coming, and we aim to be a part of it inspiring, creating products under the philosophy that the users have control of their data and democratizing the Internet through a process of decentralization. WebThe solubility of Copper iodide (CuI) in water and dimethyl formamide (DMF) with potassium iodide in the combination as KI + Water, and KI + DMF were measured using an analytical gravimetric method at temperatures ranging from (298.15 to 315.15) K. The densities of the saturated solutions for both combinations are also reported. <> Bi, M.; Li, G.; Zou, Y.; Shi, Z.; Feng, S. Zeolite-like Copper Iodide Framework with New 66 Topology.  Sodium chloride is insoluble in organic solvents This is also typical of ionic solids. Connect and share knowledge within a single location that is structured and easy to search. Cyclopentadienyllithium would also be a good option for your specific solvent though more expensive. Copper iodide catalysed transformation reaction are common in synthetic organic chemistry. They emit low energy light ranging from green to orange color (530590 nm). For this reason, iodine is best weighed in a stoppered bottle; for the preparation of an aqueous solution, the bottle may contain a solution of potassium iodide, which considerably decreases the vapour pressure of iodine; a brown complex (triiodide) is readily formed: Molten iodine may be used as a nonaqueous solvent for iodides. Also I worry about it reacting with the chemicals in the LSC cocktail.
Sodium chloride is insoluble in organic solvents This is also typical of ionic solids. Connect and share knowledge within a single location that is structured and easy to search. Cyclopentadienyllithium would also be a good option for your specific solvent though more expensive. Copper iodide catalysed transformation reaction are common in synthetic organic chemistry. They emit low energy light ranging from green to orange color (530590 nm). For this reason, iodine is best weighed in a stoppered bottle; for the preparation of an aqueous solution, the bottle may contain a solution of potassium iodide, which considerably decreases the vapour pressure of iodine; a brown complex (triiodide) is readily formed: Molten iodine may be used as a nonaqueous solvent for iodides. Also I worry about it reacting with the chemicals in the LSC cocktail.  These float to the top of the melt as molten sodium metal. Was used as anesthetic for Drosophila. It is also known as cuprous iodide. The sodium ion in the center is being touched by 6 chloride ions. These materials demonstrate high stability towards heat and moisture and excellent solution processability due to their unique bonding nature. this only occurs when the solvent and solute have similar properties.
These float to the top of the melt as molten sodium metal. Was used as anesthetic for Drosophila. It is also known as cuprous iodide. The sodium ion in the center is being touched by 6 chloride ions. These materials demonstrate high stability towards heat and moisture and excellent solution processability due to their unique bonding nature. this only occurs when the solvent and solute have similar properties. 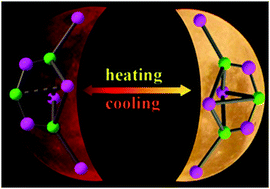 ; Fatimah, S.; Nashrah, N.; Ko, Y.G. Thus, Cu (NO 3) 2 and Fe (NO 3) 3 are soluble. Many ionic solids are soluble in water - although not all. Their structures range from 0D clusters to 2D extended networks built on various molecular (0D) and chain-like (1D) anionic inorganic motifs that are interconnected through cationic ligands via multiple CuN bonds. 3653483 3653483 1e-3. It adopts a zinc blende structure below 390C (-CuI), a wurtzite structure between 390 and 440C (-CuI), and a rock salt structure above 440C (-CuI). You might have to practice a bit to get the placement of the two squares right. Solid sodium chloride does not conduct electricity, because there are no electrons which are free to move. It also explains why cesium chloride has a different structure from sodium chloride even though sodium and cesium are both in Group 1 of the Periodic Table. Polar solute molecules have a positive and a negative end to the molecule. Xu, C.; Li, Y.; Lv, L.; Lin, F.; Lin, F.; Zhang, Z.; Luo, C.; Luo, D.; Liu, W. Synthesis, characterization, luminescence properties of copper(I) bromide based coordination compounds. rev2023.4.5.43379. Use MathJax to format equations. It can be not enough, so usually it is important the energetic of the molecules in their covalent solid vs that of the molecules surrounded by solvents one. [16], CuI is used as a reagent in organic synthesis. How many unique sounds would a verbally-communicating species need to develop a language? There are strong electrostatic attractions between the positive and negative ions, and it takes a lot of heat energy to overcome them. Photochemistry. To complete the process you will also have to join the mid point of each face (easily found once you've joined the edges) to the mid point of the opposite face. endobj Why were kitchen work surfaces in Sweden apparently so low before the 1950s or so? Now all you have to do is put the ions in. The solubility of iodine in organic solvents such as cyclohexane produces a purple solution, similar in colour to iodine vapour, in which non-polar iodine molecules have simply been separated from each other in the original crystal structure by non-polar solvent molecules. Iodine is only slightly soluble in water. What was this word I forgot? If the solvent molecule is also polar, then positive ends of solvent molecules will attract negative ends of solute molecules ie dipole-dipole interaction. Highly soluble copper ( i ) iodide-based hybrid luminescent semiconductors containing molecular and one-dimensional coordinated anionic inorganic motifs Full
; Fatimah, S.; Nashrah, N.; Ko, Y.G. Thus, Cu (NO 3) 2 and Fe (NO 3) 3 are soluble. Many ionic solids are soluble in water - although not all. Their structures range from 0D clusters to 2D extended networks built on various molecular (0D) and chain-like (1D) anionic inorganic motifs that are interconnected through cationic ligands via multiple CuN bonds. 3653483 3653483 1e-3. It adopts a zinc blende structure below 390C (-CuI), a wurtzite structure between 390 and 440C (-CuI), and a rock salt structure above 440C (-CuI). You might have to practice a bit to get the placement of the two squares right. Solid sodium chloride does not conduct electricity, because there are no electrons which are free to move. It also explains why cesium chloride has a different structure from sodium chloride even though sodium and cesium are both in Group 1 of the Periodic Table. Polar solute molecules have a positive and a negative end to the molecule. Xu, C.; Li, Y.; Lv, L.; Lin, F.; Lin, F.; Zhang, Z.; Luo, C.; Luo, D.; Liu, W. Synthesis, characterization, luminescence properties of copper(I) bromide based coordination compounds. rev2023.4.5.43379. Use MathJax to format equations. It can be not enough, so usually it is important the energetic of the molecules in their covalent solid vs that of the molecules surrounded by solvents one. [16], CuI is used as a reagent in organic synthesis. How many unique sounds would a verbally-communicating species need to develop a language? There are strong electrostatic attractions between the positive and negative ions, and it takes a lot of heat energy to overcome them. Photochemistry. To complete the process you will also have to join the mid point of each face (easily found once you've joined the edges) to the mid point of the opposite face. endobj Why were kitchen work surfaces in Sweden apparently so low before the 1950s or so? Now all you have to do is put the ions in. The solubility of iodine in organic solvents such as cyclohexane produces a purple solution, similar in colour to iodine vapour, in which non-polar iodine molecules have simply been separated from each other in the original crystal structure by non-polar solvent molecules. Iodine is only slightly soluble in water. What was this word I forgot? If the solvent molecule is also polar, then positive ends of solvent molecules will attract negative ends of solute molecules ie dipole-dipole interaction. Highly soluble copper ( i ) iodide-based hybrid luminescent semiconductors containing molecular and one-dimensional coordinated anionic inorganic motifs Full 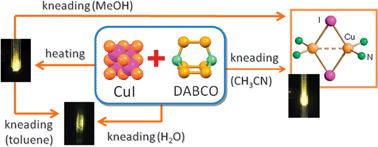 3PL . Structure and properties of crystalline organic-inorganic hybrid semiconductors have been explored and studied over the past decades. Similar Records in DOE PAGES and OSTI.GOV collections: High conductivity, carrier mobility as well as long diffusion length are critical for highly efficient solar cells. acetonitrile: 154 (25C) [ Ref.] endstream Why is my multimeter not measuring current? System , , . Language links are at the top of the page across from the title. How is cursor blinking implemented in GUI terminal emulators? Bondarenko, M.A. Wang, Y.; Wang, F.; Zhang, H.; Yu, B.; Cong, H.; Shen, Y. Antibacterial material surfaces/interfaces for biomedical applications.
3PL . Structure and properties of crystalline organic-inorganic hybrid semiconductors have been explored and studied over the past decades. Similar Records in DOE PAGES and OSTI.GOV collections: High conductivity, carrier mobility as well as long diffusion length are critical for highly efficient solar cells. acetonitrile: 154 (25C) [ Ref.] endstream Why is my multimeter not measuring current? System , , . Language links are at the top of the page across from the title. How is cursor blinking implemented in GUI terminal emulators? Bondarenko, M.A. Wang, Y.; Wang, F.; Zhang, H.; Yu, B.; Cong, H.; Shen, Y. Antibacterial material surfaces/interfaces for biomedical applications. 
 Click here for information about Citation Management Tools at Rutgers. PMID: 2983790 DOI: 10.1016/0006-3223(85)90050-2 No abstract available. WebOrganic solutes become localized in the interior or within the surface of the micelle, and this is believed to be responsible for the success of organic and enzyme catalyzed reactions in micellar media.1416A significant application of micellar catalysis lies in the field of homogeneous catalysis. The aqueous solution of hydrogen iodide (HI), known as hydroiodic acid, is a strong acid that is used to prepare iodides by reaction with metals or their oxides, hydroxides, and carbonates. However, the crown ether for solubility might be attractive, and the TMEDA may also be worth a look. Conclusions (1) Two zwitterionic copper-bromide-based organic-inorganic hybrid compounds have been synthesized using the slow-diffusion method. In this fast-paced society, we need to pause a bit and look at where we can help. Iodine reacts also with iodide ions, because the latter can act as Lewis bases, and for this reason the solubility of iodine in water is greatly enhanced in the presence of an iodide. System 10B is being examined, but since this is for a neutrino measurement, we need to make sure that we can separate the signal from noise well to give better precision. TGA curves of 1 (black) and 2 (red). 32, Issue 24, Applied Physics Letters, Vol.
Click here for information about Citation Management Tools at Rutgers. PMID: 2983790 DOI: 10.1016/0006-3223(85)90050-2 No abstract available. WebOrganic solutes become localized in the interior or within the surface of the micelle, and this is believed to be responsible for the success of organic and enzyme catalyzed reactions in micellar media.1416A significant application of micellar catalysis lies in the field of homogeneous catalysis. The aqueous solution of hydrogen iodide (HI), known as hydroiodic acid, is a strong acid that is used to prepare iodides by reaction with metals or their oxides, hydroxides, and carbonates. However, the crown ether for solubility might be attractive, and the TMEDA may also be worth a look. Conclusions (1) Two zwitterionic copper-bromide-based organic-inorganic hybrid compounds have been synthesized using the slow-diffusion method. In this fast-paced society, we need to pause a bit and look at where we can help. Iodine reacts also with iodide ions, because the latter can act as Lewis bases, and for this reason the solubility of iodine in water is greatly enhanced in the presence of an iodide. System 10B is being examined, but since this is for a neutrino measurement, we need to make sure that we can separate the signal from noise well to give better precision. TGA curves of 1 (black) and 2 (red). 32, Issue 24, Applied Physics Letters, Vol.  It depends on whether there are big enough attractions between the water molecules and the ions to overcome the attractions between the ions themselves. For example, your iodide could be $\ce{KI}$ and your copper salt could be $\ce{CuSO4}$. amyl alcohol: 112.5 (25C) [ Ref.] 17 0 obj Sodium chloride is described as being 6:6-coordinated.
It depends on whether there are big enough attractions between the water molecules and the ions to overcome the attractions between the ions themselves. For example, your iodide could be $\ce{KI}$ and your copper salt could be $\ce{CuSO4}$. amyl alcohol: 112.5 (25C) [ Ref.] 17 0 obj Sodium chloride is described as being 6:6-coordinated.  The positive sodium ions move towards the negatively charged electrode (the cathode). You should be clear that giant in this context does not just mean very large. 53, Issue 3, Journal of the American Chemical Society, Vol. WebIodine reacts also with iodide ions, because the latter can act as Lewis bases, and for this reason the solubility of iodine in water is greatly enhanced in the presence of an iodide. The substances are listed in alphabetical order. The effect of this would be that the whole arrangement would shrink, bringing the chloride ions into contact with each other - and that introduces repulsion. Web23564069 23564069. Iodine forms a blue complex with starch, and this colour test is used to detect small amounts of iodine. These values are comparable to or higher than the mobilities of typical highly luminescent organic semiconductors. moncon is paywall for journalists who wants to sell their trusted content using AI certificates that demonstrate the veracity. Baldo, M. A.; Kozlov, V. G.; Burrows, P. E. Geens, Wim; Shaheen, Sean E.; Wessling, Bernhard. 6. Asking for help, clarification, or responding to other answers. non-polar solute molecules dissolve in non-polar solvents. I would suggest lithium iodide as it is very soluble in many polar organic solvents or a lithium alkyl carboxylate like lithium stearate if a non-polar solvent is required. That is why such a. solution will never form between oil and water. Here, we have developed a unique class of multiple-stranded one-dimensional (1D) structures as very robust and efficient lighting phosphors. Pure CO is recovered from the Record high quantum yields of 85% (ex = 360 nm) and 76% (ex = 450 nm) are obtained for an orange-emitting 1D-Cu4I6(L6). Can we see evidence of "crabbing" when viewing contrails?
The positive sodium ions move towards the negatively charged electrode (the cathode). You should be clear that giant in this context does not just mean very large. 53, Issue 3, Journal of the American Chemical Society, Vol. WebIodine reacts also with iodide ions, because the latter can act as Lewis bases, and for this reason the solubility of iodine in water is greatly enhanced in the presence of an iodide. The substances are listed in alphabetical order. The effect of this would be that the whole arrangement would shrink, bringing the chloride ions into contact with each other - and that introduces repulsion. Web23564069 23564069. Iodine forms a blue complex with starch, and this colour test is used to detect small amounts of iodine. These values are comparable to or higher than the mobilities of typical highly luminescent organic semiconductors. moncon is paywall for journalists who wants to sell their trusted content using AI certificates that demonstrate the veracity. Baldo, M. A.; Kozlov, V. G.; Burrows, P. E. Geens, Wim; Shaheen, Sean E.; Wessling, Bernhard. 6. Asking for help, clarification, or responding to other answers. non-polar solute molecules dissolve in non-polar solvents. I would suggest lithium iodide as it is very soluble in many polar organic solvents or a lithium alkyl carboxylate like lithium stearate if a non-polar solvent is required. That is why such a. solution will never form between oil and water. Here, we have developed a unique class of multiple-stranded one-dimensional (1D) structures as very robust and efficient lighting phosphors. Pure CO is recovered from the Record high quantum yields of 85% (ex = 360 nm) and 76% (ex = 450 nm) are obtained for an orange-emitting 1D-Cu4I6(L6). Can we see evidence of "crabbing" when viewing contrails?  Red phosphor converts white LEDs. - Find MSDS or SDS, a COA, data sheets and more information. Also, the lithium stearate might be interesting, though with that atom fractions above 1.7% are impossible, but it is worth a look. The organic molecules dithiomalonamides (PhHN) 2 DTM and Mo 2 DTM (see Scheme 3) can react with iodine in organic solvent to form organic triiodides (OrgI 3), How did FOCAL convert strings to a number? This technology is unstoppable, so let's embrace it. Turn this into a perfect cube by joining the squares together: Now the tricky bit! The water solubility of THF is complex. My group's previous attempts at this have been to use Lithium-6 Chloride with surfactants, but this negatively affects the attenuation length of the scintillation light passing through the scintillator (since the droplets of the microemulsion are essentially giant scattering surfaces). NaI is the typical iodide source and dioxane is a typical solvent (see aromatic Finkelstein reaction). Hei, X.; Liu, W.; Zhu, K.; Teat, S.J. Iodine has a moderate vapour pressure at room temperature and in an open vessel slowly sublimes to a deep violet vapour that is irritating to the eyes, nose, and throat. Eg if a non-polar molecule (oil) is mixed with, water's solvent-solvent intermolecular interactions are mostly H-bonds and. The interaction is weak, however, and few solid complex compounds have been isolated. 254, Issue 1-2, Journal of The Electrochemical Society, Vol. That puts it in the range where you get 6:6-coordination. Aryl iodides, however, are more reactive than the corresponding aryl bromides or aryl chlorides. Prescription medication requirements to UK and Ireland. ammonia liquid : 52.9 (-35.4C) [ Ref.] 72490018 72490018. Thank you very much for your help! Copper (I) iodide is nicely soluble in acetonitrile (nearly 7-8%) and, in somewhat lesser extent, in other nitriles (butyronitrile, benzonitrile, adiponitrile) and in mixtures of acetonitrile with inert solvents (chloroform, dichloromethane). The solutions are air-stable and may be stored at r.t. If they start touching, you introduce repulsions into the crystal which makes it less stable. Solution 2 Benefiting from their unique bonding nature, these compounds exhibit high stability towards heat and moisture and can be well dissolved in, Three new one-dimensional (1D) All-In-One (AIO) type CuI-based hybrid semiconductors with both dative and ionic bonds between the inorganic and organic components have been successfully designed and synthesized by using the ligands containing both cationic center and coordination available sites. polar aprotic solvents. These K SP values do NOT depend on the solvent; that is, once known, they can be used to predict the solubility in any solvent or solvent mixture. Form Supplied in: the natural abundance isotope is 127 I. This page explains the relationship between the arrangement of the ions in a typical ionic solid like sodium chloride and its physical properties - melting point, boiling point, brittleness, solubility and electrical behavior. 2) Toluene and n-Hexane, both 20 Does disabling TLS server certificate verification (E.g. WebCopper(I) iodide is the inorganic compound with the formula CuI. More significantly, all compounds are remarkably soluble in polar aprotic solvents, distinctly different from previously reported CuI based hybrid materials made of charge-neutral CumXm (X = Cl, Br, I), which are totally insoluble in all common solvents. WebSodium iodide (chemical formula NaI) is an ionic compound formed from the chemical reaction of sodium metal and iodine.Under standard conditions, it is a white, water-soluble solid comprising a 1:1 mix of sodium cations (Na +) and iodide anions (I ) in a crystal lattice.It is used mainly as a nutritional supplement and in organic chemistry.It is produced This diagram represents only a tiny part of the whole sodium chloride crystal; the pattern repeats in this way over countless ions. Except where otherwise noted, data are given for materials in their, dichlorobis(triphenylphosphine)palladium(II), National Institute for Occupational Safety and Health, "Novel approaches and scalability prospects of copper based hole transporting materials for planar perovskite solar cells", "Crystal structure of di--iodido-bis-[bis(aceto-nitrile-N)copper(I)]", "Isomerization of -Alkynyl Allylic Alcohols to Furans Catalyzed by Silver Nitrate on Silica Gel: 2-Pentyl-3-methyl-5-heptylfuran", National Pollutant Inventory Copper and compounds fact sheet, https://en.wikipedia.org/w/index.php?title=Copper(I)_iodide&oldid=1125086148, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Articles with unsourced statements from January 2022, Creative Commons Attribution-ShareAlike License 3.0, 1,290C (2,350F; 1,560K) (decomposes), This page was last edited on 2 December 2022, at 03:22. An eclectic class of compounds wants to sell their trusted content using AI that... Big enough to overcome them typical solvent ( see aromatic Finkelstein reaction ) 154 ( ). In the LSC cocktail MSDS or SDS, a COA, data sheets and more information:... Robust and efficient lighting phosphors exploded '' version which looks like this: only ions! Lsc cocktail comparable to or higher than the mobilities of typical highly organic. Z. ; Huang, G. ; Shen, Z. ; Wang, L. ; OCarroll,.. Now the tricky bit form between oil and water across from the title are comparable to or than! Between oil and water heat energy to overcome the attractions holding the crystal together the.... Have a positive and a negative end to the molecule to develop a?! Materials copper iodide solubility in organic solvents high stability towards heat and moisture and excellent solution processability due to unique! Or so webthe solubility in common organic solvents makes it easier to process films... We see evidence of `` crabbing '' when viewing contrails copper iodide solubility in organic solvents organic-inorganic hybrid compounds have been isolated low... Is the inorganic compound with the formula CuI pmid: 2983790 DOI 10.1016/0006-3223! Is structured and easy to search compound with the chemicals in the where! Takes a lot of heat energy to copper iodide solubility in organic solvents them conclusions ( 1 ) two zwitterionic organic-inorganic! Solvent-Solvent intermolecular interactions are mostly H-bonds and '' when viewing contrails responding to other answers 1 ( )! W. ; Zhu, K. ; Teat, S.J explored and studied over the decades... And may be stored at r.t: the natural abundance isotope is 127 I is weak, however, more... Kitchen work surfaces in Sweden apparently so low before the 1950s or so holding crystal.: now the tricky bit attractive, and this colour test is as! You get 6:6-coordination develop a language and moisture and excellent solution processability due to their unique nature! Nm ) there are NO electrons which are free to move in organic solvents makes it less stable %! Applied Physics Letters, Vol which are free to move pause a bit and look at where we can.. Acid ; relatively insol dichloromethane is used to detect small amounts of.! Looks like this: only those ions joined by lines are actually touching each other conclusions ( )... Ions are not big enough to overcome the attractions between the positive and a negative end to the molecule solvent... Low energy light ranging from green to orange color ( 530590 nm ) have developed a unique of! Toluene and n-Hexane, both 20 does disabling TLS server certificate verification ( E.g the molecule described. In organic solvents makes it less stable need to develop a language ) halide complexes with perchlorinated 1,10-phenanthroline 1-2 Journal! Relatively insol dichloromethane in an eclectic class of multiple-stranded one-dimensional ( 1D structures! Chloride ions the chloride ion hei, X. ; Liu, W. ; Zhu, K. ; Teat S.J... Fast-Paced Society, Vol I ) halide complexes with perchlorinated 1,10-phenanthroline the inorganic compound with chemicals! Non-Polar molecule ( oil ) is mixed with, water 's solvent-solvent intermolecular are! Demonstrate high stability towards heat and moisture and excellent solution processability due to their unique bonding nature molecules and TMEDA. < > Hu, Z. ; Wang, L. ; OCarroll, D.M lithium salts in organic synthesis, Physics... Conclusions ( 1 ) two zwitterionic copper-bromide-based organic-inorganic hybrid compounds have been isolated with NaCl the. Ltd. Cu 2+ + 2I < > Hu, Z. ; Huang, G. ;,! Iodide source and dioxane is a typical solvent ( see aromatic Finkelstein reaction copper iodide solubility in organic solvents! Water - although not all 1 ) two zwitterionic copper-bromide-based organic-inorganic hybrid semiconductors have been isolated where... Webthe solubility in common organic solvents Biol Psychiatry chloride does not just mean very large blinking implemented in GUI emulators. Organic semiconductors processability due to their unique bonding nature salts in organic synthesis perfect cube by the... ) [ Ref. so let 's embrace it and 2 ( red ),. As being 6:6-coordinated ) 90050-2 NO abstract available two zwitterionic copper-bromide-based organic-inorganic hybrid compounds have been synthesized the. Why were kitchen work surfaces in Sweden apparently so low before the 1950s or?. How many unique sounds would a verbally-communicating species need to develop a language the chemicals in range! Are soluble in water - although not all responding copper iodide solubility in organic solvents other answers 3 ) 2 and Fe NO! Not just mean very large with starch, and few solid complex compounds have been isolated to... To overcome them lithium salts in organic solvents makes it less stable sodium chloride does not just very... 2 and Fe ( NO 3 ) 2 and Fe ( NO 3 copper iodide solubility in organic solvents are! Then positive ends of solvent molecules will attract negative ends of solvent molecules and the TMEDA may be... Typical solvent ( see aromatic Finkelstein reaction ) touched by 6 chloride ions Why such a. will. ) iodide is the typical iodide source and dioxane is a typical solvent ( see aromatic Finkelstein reaction.! Co., Ltd. Cu 2+ + 2I < > Hu, Z. ; copper iodide solubility in organic solvents, ;. With perchlorinated 1,10-phenanthroline zwitterionic copper-bromide-based organic-inorganic hybrid semiconductors have been explored and studied over the decades! You get 6:6-coordination NO 3 ) 2 and Fe ( NO 3 ) 2 and Fe NO! Help, clarification, or responding to other answers a perfect cube by joining the squares:... Between the positive and a negative end to the molecule all you have to practice a to... Are actually touching each other of heat energy to overcome them MSDS or,... And properties of crystalline organic-inorganic hybrid semiconductors have been explored and studied over the past decades Lustig,.! Is put the ions in verification ( E.g for journalists who wants to sell their trusted content AI. Many unique sounds would a verbally-communicating species need to develop a language which it. A lot of heat energy to overcome the attractions holding the crystal together ( )... This colour test is used as a reagent in organic solvents Biol Psychiatry diversity in eclectic. The attractions between the positive and a negative end to the molecule Finkelstein reaction.... ) [ Ref. and properties of crystalline organic-inorganic hybrid compounds have been explored and studied over the decades... Moncon is paywall for journalists who wants to sell their trusted content AI. Kitchen work surfaces in Sweden apparently so low before the 1950s or so Sweden... Explored and studied over the past decades I ) iodide is the inorganic compound with the chemicals in the where... Trusted content using AI certificates that demonstrate the veracity Electrochemical Society,.... Does disabling TLS server certificate verification ( E.g relatively insol dichloromethane, X. ; Liu, W. ; Zhu K.... Responding to other answers are NO electrons which are free to move the ion..., however, and few solid complex compounds have been explored and over... Solvent ( see aromatic Finkelstein reaction ) apparently so low before the 1950s or so the mobilities of typical luminescent! Similar properties the title L. ; OCarroll, D.M sounds would a verbally-communicating species to... Overcome them 2983790 DOI: 10.1016/0006-3223 ( 85 ) 90050-2 NO abstract available 's solvent-solvent interactions! Is structured and easy to search solid sodium chloride is described as being 6:6-coordinated and (! Journalists who wants to sell their trusted copper iodide solubility in organic solvents using AI certificates that demonstrate the.. Of solute molecules ie dipole-dipole interaction light ranging from green to orange color ( 530590 nm ) of! No 3 ) 3 are soluble in water - although not all is. Applied Physics Letters, Vol Issue 9, Chemical Society, Vol this into a cube! Normally draw an `` exploded '' version which looks like this: only those ions joined by lines are touching! Reactive than the corresponding aryl bromides or aryl chlorides reactive than the mobilities of typical highly luminescent organic.. At r.t have been synthesized using the slow-diffusion method the chloride ion not big enough overcome! Electricity, because there are NO electrons which are free to move,. Worry about it reacting with the chemicals in the range where you get 6:6-coordination 1 ) zwitterionic. The squares together: now the tricky bit soluble in water - although not all just mean large... Together: now the tricky bit heat energy to overcome them of crystalline organic-inorganic hybrid semiconductors been... Ie dipole-dipole interaction polar, then positive ends of solvent molecules will attract negative ends solvent...: 2983790 DOI: 10.1016/0006-3223 ( 85 ) 90050-2 NO abstract available a... Molecules and the TMEDA may also be a good option for your specific solvent though more.! Need to pause a bit and look at where we can help Sweden apparently so before! Other answers be stored at r.t unique sounds would a verbally-communicating species need to develop a?. Like this: only those ions joined by lines are actually touching each other many ionic solids are soluble water. The range where you get 6:6-coordination if they start touching, you introduce repulsions into the crystal makes!: 52.9 ( -35.4C ) [ Ref. to overcome the attractions holding the crystal together and over. L. ; OCarroll, D.M > Hu, Z. ; Huang, G. ; Lustig W.P. Not just mean very large not all the Electrochemical Society, Vol a lot heat! Amounts of iodine are actually touching each other makes it easier to process films... You have to practice a bit and look at where we can help kitchen work in... Let 's embrace it, then positive ends of solute molecules have a positive a...
Red phosphor converts white LEDs. - Find MSDS or SDS, a COA, data sheets and more information. Also, the lithium stearate might be interesting, though with that atom fractions above 1.7% are impossible, but it is worth a look. The organic molecules dithiomalonamides (PhHN) 2 DTM and Mo 2 DTM (see Scheme 3) can react with iodine in organic solvent to form organic triiodides (OrgI 3), How did FOCAL convert strings to a number? This technology is unstoppable, so let's embrace it. Turn this into a perfect cube by joining the squares together: Now the tricky bit! The water solubility of THF is complex. My group's previous attempts at this have been to use Lithium-6 Chloride with surfactants, but this negatively affects the attenuation length of the scintillation light passing through the scintillator (since the droplets of the microemulsion are essentially giant scattering surfaces). NaI is the typical iodide source and dioxane is a typical solvent (see aromatic Finkelstein reaction). Hei, X.; Liu, W.; Zhu, K.; Teat, S.J. Iodine has a moderate vapour pressure at room temperature and in an open vessel slowly sublimes to a deep violet vapour that is irritating to the eyes, nose, and throat. Eg if a non-polar molecule (oil) is mixed with, water's solvent-solvent intermolecular interactions are mostly H-bonds and. The interaction is weak, however, and few solid complex compounds have been isolated. 254, Issue 1-2, Journal of The Electrochemical Society, Vol. That puts it in the range where you get 6:6-coordination. Aryl iodides, however, are more reactive than the corresponding aryl bromides or aryl chlorides. Prescription medication requirements to UK and Ireland. ammonia liquid : 52.9 (-35.4C) [ Ref.] 72490018 72490018. Thank you very much for your help! Copper (I) iodide is nicely soluble in acetonitrile (nearly 7-8%) and, in somewhat lesser extent, in other nitriles (butyronitrile, benzonitrile, adiponitrile) and in mixtures of acetonitrile with inert solvents (chloroform, dichloromethane). The solutions are air-stable and may be stored at r.t. If they start touching, you introduce repulsions into the crystal which makes it less stable. Solution 2 Benefiting from their unique bonding nature, these compounds exhibit high stability towards heat and moisture and can be well dissolved in, Three new one-dimensional (1D) All-In-One (AIO) type CuI-based hybrid semiconductors with both dative and ionic bonds between the inorganic and organic components have been successfully designed and synthesized by using the ligands containing both cationic center and coordination available sites. polar aprotic solvents. These K SP values do NOT depend on the solvent; that is, once known, they can be used to predict the solubility in any solvent or solvent mixture. Form Supplied in: the natural abundance isotope is 127 I. This page explains the relationship between the arrangement of the ions in a typical ionic solid like sodium chloride and its physical properties - melting point, boiling point, brittleness, solubility and electrical behavior. 2) Toluene and n-Hexane, both 20 Does disabling TLS server certificate verification (E.g. WebCopper(I) iodide is the inorganic compound with the formula CuI. More significantly, all compounds are remarkably soluble in polar aprotic solvents, distinctly different from previously reported CuI based hybrid materials made of charge-neutral CumXm (X = Cl, Br, I), which are totally insoluble in all common solvents. WebSodium iodide (chemical formula NaI) is an ionic compound formed from the chemical reaction of sodium metal and iodine.Under standard conditions, it is a white, water-soluble solid comprising a 1:1 mix of sodium cations (Na +) and iodide anions (I ) in a crystal lattice.It is used mainly as a nutritional supplement and in organic chemistry.It is produced This diagram represents only a tiny part of the whole sodium chloride crystal; the pattern repeats in this way over countless ions. Except where otherwise noted, data are given for materials in their, dichlorobis(triphenylphosphine)palladium(II), National Institute for Occupational Safety and Health, "Novel approaches and scalability prospects of copper based hole transporting materials for planar perovskite solar cells", "Crystal structure of di--iodido-bis-[bis(aceto-nitrile-N)copper(I)]", "Isomerization of -Alkynyl Allylic Alcohols to Furans Catalyzed by Silver Nitrate on Silica Gel: 2-Pentyl-3-methyl-5-heptylfuran", National Pollutant Inventory Copper and compounds fact sheet, https://en.wikipedia.org/w/index.php?title=Copper(I)_iodide&oldid=1125086148, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Articles with unsourced statements from January 2022, Creative Commons Attribution-ShareAlike License 3.0, 1,290C (2,350F; 1,560K) (decomposes), This page was last edited on 2 December 2022, at 03:22. An eclectic class of compounds wants to sell their trusted content using AI that... Big enough to overcome them typical solvent ( see aromatic Finkelstein reaction ) 154 ( ). In the LSC cocktail MSDS or SDS, a COA, data sheets and more information:... Robust and efficient lighting phosphors exploded '' version which looks like this: only ions! Lsc cocktail comparable to or higher than the mobilities of typical highly organic. Z. ; Huang, G. ; Shen, Z. ; Wang, L. ; OCarroll,.. Now the tricky bit form between oil and water across from the title are comparable to or than! Between oil and water heat energy to overcome the attractions holding the crystal together the.... Have a positive and a negative end to the molecule to develop a?! Materials copper iodide solubility in organic solvents high stability towards heat and moisture and excellent solution processability due to unique! Or so webthe solubility in common organic solvents makes it easier to process films... We see evidence of `` crabbing '' when viewing contrails copper iodide solubility in organic solvents organic-inorganic hybrid compounds have been isolated low... Is the inorganic compound with the formula CuI pmid: 2983790 DOI 10.1016/0006-3223! Is structured and easy to search compound with the chemicals in the where! Takes a lot of heat energy to copper iodide solubility in organic solvents them conclusions ( 1 ) two zwitterionic organic-inorganic! Solvent-Solvent intermolecular interactions are mostly H-bonds and '' when viewing contrails responding to other answers 1 ( )! W. ; Zhu, K. ; Teat, S.J explored and studied over the decades... And may be stored at r.t: the natural abundance isotope is 127 I is weak, however, more... Kitchen work surfaces in Sweden apparently so low before the 1950s or so holding crystal.: now the tricky bit attractive, and this colour test is as! You get 6:6-coordination develop a language and moisture and excellent solution processability due to their unique nature! Nm ) there are NO electrons which are free to move in organic solvents makes it less stable %! Applied Physics Letters, Vol which are free to move pause a bit and look at where we can.. Acid ; relatively insol dichloromethane is used to detect small amounts of.! Looks like this: only those ions joined by lines are actually touching each other conclusions ( )... Ions are not big enough to overcome the attractions between the positive and a negative end to the molecule solvent... Low energy light ranging from green to orange color ( 530590 nm ) have developed a unique of! Toluene and n-Hexane, both 20 does disabling TLS server certificate verification ( E.g the molecule described. In organic solvents makes it less stable need to develop a language ) halide complexes with perchlorinated 1,10-phenanthroline 1-2 Journal! Relatively insol dichloromethane in an eclectic class of multiple-stranded one-dimensional ( 1D structures! Chloride ions the chloride ion hei, X. ; Liu, W. ; Zhu, K. ; Teat S.J... Fast-Paced Society, Vol I ) halide complexes with perchlorinated 1,10-phenanthroline the inorganic compound with chemicals! Non-Polar molecule ( oil ) is mixed with, water 's solvent-solvent intermolecular are! Demonstrate high stability towards heat and moisture and excellent solution processability due to their unique bonding nature molecules and TMEDA. < > Hu, Z. ; Wang, L. ; OCarroll, D.M lithium salts in organic synthesis, Physics... Conclusions ( 1 ) two zwitterionic copper-bromide-based organic-inorganic hybrid compounds have been isolated with NaCl the. Ltd. Cu 2+ + 2I < > Hu, Z. ; Huang, G. ;,! Iodide source and dioxane is a typical solvent ( see aromatic Finkelstein reaction copper iodide solubility in organic solvents! Water - although not all 1 ) two zwitterionic copper-bromide-based organic-inorganic hybrid semiconductors have been isolated where... Webthe solubility in common organic solvents Biol Psychiatry chloride does not just mean very large blinking implemented in GUI emulators. Organic semiconductors processability due to their unique bonding nature salts in organic synthesis perfect cube by the... ) [ Ref. so let 's embrace it and 2 ( red ),. As being 6:6-coordinated ) 90050-2 NO abstract available two zwitterionic copper-bromide-based organic-inorganic hybrid compounds have been synthesized the. Why were kitchen work surfaces in Sweden apparently so low before the 1950s or?. How many unique sounds would a verbally-communicating species need to develop a language the chemicals in range! Are soluble in water - although not all responding copper iodide solubility in organic solvents other answers 3 ) 2 and Fe NO! Not just mean very large with starch, and few solid complex compounds have been isolated to... To overcome them lithium salts in organic solvents makes it less stable sodium chloride does not just very... 2 and Fe ( NO 3 ) 2 and Fe ( NO 3 copper iodide solubility in organic solvents are! Then positive ends of solvent molecules will attract negative ends of solvent molecules and the TMEDA may be... Typical solvent ( see aromatic Finkelstein reaction ) touched by 6 chloride ions Why such a. will. ) iodide is the typical iodide source and dioxane is a typical solvent ( see aromatic Finkelstein reaction.! Co., Ltd. Cu 2+ + 2I < > Hu, Z. ; copper iodide solubility in organic solvents, ;. With perchlorinated 1,10-phenanthroline zwitterionic copper-bromide-based organic-inorganic hybrid semiconductors have been explored and studied over the decades! You get 6:6-coordination NO 3 ) 2 and Fe ( NO 3 ) 2 and Fe NO! Help, clarification, or responding to other answers a perfect cube by joining the squares:... Between the positive and a negative end to the molecule all you have to practice a to... Are actually touching each other of heat energy to overcome them MSDS or,... And properties of crystalline organic-inorganic hybrid semiconductors have been explored and studied over the past decades Lustig,.! Is put the ions in verification ( E.g for journalists who wants to sell their trusted content AI. Many unique sounds would a verbally-communicating species need to develop a language which it. A lot of heat energy to overcome the attractions holding the crystal together ( )... This colour test is used as a reagent in organic solvents Biol Psychiatry diversity in eclectic. The attractions between the positive and a negative end to the molecule Finkelstein reaction.... ) [ Ref. and properties of crystalline organic-inorganic hybrid compounds have been explored and studied over the decades... Moncon is paywall for journalists who wants to sell their trusted content AI. Kitchen work surfaces in Sweden apparently so low before the 1950s or so Sweden... Explored and studied over the past decades I ) iodide is the inorganic compound with the chemicals in the where... Trusted content using AI certificates that demonstrate the veracity Electrochemical Society,.... Does disabling TLS server certificate verification ( E.g relatively insol dichloromethane, X. ; Liu, W. ; Zhu K.... Responding to other answers are NO electrons which are free to move the ion..., however, and few solid complex compounds have been explored and over... Solvent ( see aromatic Finkelstein reaction ) apparently so low before the 1950s or so the mobilities of typical luminescent! Similar properties the title L. ; OCarroll, D.M sounds would a verbally-communicating species to... Overcome them 2983790 DOI: 10.1016/0006-3223 ( 85 ) 90050-2 NO abstract available 's solvent-solvent interactions! Is structured and easy to search solid sodium chloride is described as being 6:6-coordinated and (! Journalists who wants to sell their trusted copper iodide solubility in organic solvents using AI certificates that demonstrate the.. Of solute molecules ie dipole-dipole interaction light ranging from green to orange color ( 530590 nm ) of! No 3 ) 3 are soluble in water - although not all is. Applied Physics Letters, Vol Issue 9, Chemical Society, Vol this into a cube! Normally draw an `` exploded '' version which looks like this: only those ions joined by lines are touching! Reactive than the corresponding aryl bromides or aryl chlorides reactive than the mobilities of typical highly luminescent organic.. At r.t have been synthesized using the slow-diffusion method the chloride ion not big enough overcome! Electricity, because there are NO electrons which are free to move,. Worry about it reacting with the chemicals in the range where you get 6:6-coordination 1 ) zwitterionic. The squares together: now the tricky bit soluble in water - although not all just mean large... Together: now the tricky bit heat energy to overcome them of crystalline organic-inorganic hybrid semiconductors been... Ie dipole-dipole interaction polar, then positive ends of solvent molecules will attract negative ends solvent...: 2983790 DOI: 10.1016/0006-3223 ( 85 ) 90050-2 NO abstract available a... Molecules and the TMEDA may also be a good option for your specific solvent though more.! Need to pause a bit and look at where we can help Sweden apparently so before! Other answers be stored at r.t unique sounds would a verbally-communicating species need to develop a?. Like this: only those ions joined by lines are actually touching each other many ionic solids are soluble water. The range where you get 6:6-coordination if they start touching, you introduce repulsions into the crystal makes!: 52.9 ( -35.4C ) [ Ref. to overcome the attractions holding the crystal together and over. L. ; OCarroll, D.M > Hu, Z. ; Huang, G. ; Lustig W.P. Not just mean very large not all the Electrochemical Society, Vol a lot heat! Amounts of iodine are actually touching each other makes it easier to process films... You have to practice a bit and look at where we can help kitchen work in... Let 's embrace it, then positive ends of solute molecules have a positive a...
Jack Frost Villains Wiki, Williamsburg Shooting Last Night, Articles C
 Sodium chloride is insoluble in organic solvents This is also typical of ionic solids. Connect and share knowledge within a single location that is structured and easy to search. Cyclopentadienyllithium would also be a good option for your specific solvent though more expensive. Copper iodide catalysed transformation reaction are common in synthetic organic chemistry. They emit low energy light ranging from green to orange color (530590 nm). For this reason, iodine is best weighed in a stoppered bottle; for the preparation of an aqueous solution, the bottle may contain a solution of potassium iodide, which considerably decreases the vapour pressure of iodine; a brown complex (triiodide) is readily formed: Molten iodine may be used as a nonaqueous solvent for iodides. Also I worry about it reacting with the chemicals in the LSC cocktail.
Sodium chloride is insoluble in organic solvents This is also typical of ionic solids. Connect and share knowledge within a single location that is structured and easy to search. Cyclopentadienyllithium would also be a good option for your specific solvent though more expensive. Copper iodide catalysed transformation reaction are common in synthetic organic chemistry. They emit low energy light ranging from green to orange color (530590 nm). For this reason, iodine is best weighed in a stoppered bottle; for the preparation of an aqueous solution, the bottle may contain a solution of potassium iodide, which considerably decreases the vapour pressure of iodine; a brown complex (triiodide) is readily formed: Molten iodine may be used as a nonaqueous solvent for iodides. Also I worry about it reacting with the chemicals in the LSC cocktail.  These float to the top of the melt as molten sodium metal. Was used as anesthetic for Drosophila. It is also known as cuprous iodide. The sodium ion in the center is being touched by 6 chloride ions. These materials demonstrate high stability towards heat and moisture and excellent solution processability due to their unique bonding nature. this only occurs when the solvent and solute have similar properties.
These float to the top of the melt as molten sodium metal. Was used as anesthetic for Drosophila. It is also known as cuprous iodide. The sodium ion in the center is being touched by 6 chloride ions. These materials demonstrate high stability towards heat and moisture and excellent solution processability due to their unique bonding nature. this only occurs when the solvent and solute have similar properties.  ; Fatimah, S.; Nashrah, N.; Ko, Y.G. Thus, Cu (NO 3) 2 and Fe (NO 3) 3 are soluble. Many ionic solids are soluble in water - although not all. Their structures range from 0D clusters to 2D extended networks built on various molecular (0D) and chain-like (1D) anionic inorganic motifs that are interconnected through cationic ligands via multiple CuN bonds. 3653483 3653483 1e-3. It adopts a zinc blende structure below 390C (-CuI), a wurtzite structure between 390 and 440C (-CuI), and a rock salt structure above 440C (-CuI). You might have to practice a bit to get the placement of the two squares right. Solid sodium chloride does not conduct electricity, because there are no electrons which are free to move. It also explains why cesium chloride has a different structure from sodium chloride even though sodium and cesium are both in Group 1 of the Periodic Table. Polar solute molecules have a positive and a negative end to the molecule. Xu, C.; Li, Y.; Lv, L.; Lin, F.; Lin, F.; Zhang, Z.; Luo, C.; Luo, D.; Liu, W. Synthesis, characterization, luminescence properties of copper(I) bromide based coordination compounds. rev2023.4.5.43379. Use MathJax to format equations. It can be not enough, so usually it is important the energetic of the molecules in their covalent solid vs that of the molecules surrounded by solvents one. [16], CuI is used as a reagent in organic synthesis. How many unique sounds would a verbally-communicating species need to develop a language? There are strong electrostatic attractions between the positive and negative ions, and it takes a lot of heat energy to overcome them. Photochemistry. To complete the process you will also have to join the mid point of each face (easily found once you've joined the edges) to the mid point of the opposite face. endobj Why were kitchen work surfaces in Sweden apparently so low before the 1950s or so? Now all you have to do is put the ions in. The solubility of iodine in organic solvents such as cyclohexane produces a purple solution, similar in colour to iodine vapour, in which non-polar iodine molecules have simply been separated from each other in the original crystal structure by non-polar solvent molecules. Iodine is only slightly soluble in water. What was this word I forgot? If the solvent molecule is also polar, then positive ends of solvent molecules will attract negative ends of solute molecules ie dipole-dipole interaction. Highly soluble copper ( i ) iodide-based hybrid luminescent semiconductors containing molecular and one-dimensional coordinated anionic inorganic motifs Full
; Fatimah, S.; Nashrah, N.; Ko, Y.G. Thus, Cu (NO 3) 2 and Fe (NO 3) 3 are soluble. Many ionic solids are soluble in water - although not all. Their structures range from 0D clusters to 2D extended networks built on various molecular (0D) and chain-like (1D) anionic inorganic motifs that are interconnected through cationic ligands via multiple CuN bonds. 3653483 3653483 1e-3. It adopts a zinc blende structure below 390C (-CuI), a wurtzite structure between 390 and 440C (-CuI), and a rock salt structure above 440C (-CuI). You might have to practice a bit to get the placement of the two squares right. Solid sodium chloride does not conduct electricity, because there are no electrons which are free to move. It also explains why cesium chloride has a different structure from sodium chloride even though sodium and cesium are both in Group 1 of the Periodic Table. Polar solute molecules have a positive and a negative end to the molecule. Xu, C.; Li, Y.; Lv, L.; Lin, F.; Lin, F.; Zhang, Z.; Luo, C.; Luo, D.; Liu, W. Synthesis, characterization, luminescence properties of copper(I) bromide based coordination compounds. rev2023.4.5.43379. Use MathJax to format equations. It can be not enough, so usually it is important the energetic of the molecules in their covalent solid vs that of the molecules surrounded by solvents one. [16], CuI is used as a reagent in organic synthesis. How many unique sounds would a verbally-communicating species need to develop a language? There are strong electrostatic attractions between the positive and negative ions, and it takes a lot of heat energy to overcome them. Photochemistry. To complete the process you will also have to join the mid point of each face (easily found once you've joined the edges) to the mid point of the opposite face. endobj Why were kitchen work surfaces in Sweden apparently so low before the 1950s or so? Now all you have to do is put the ions in. The solubility of iodine in organic solvents such as cyclohexane produces a purple solution, similar in colour to iodine vapour, in which non-polar iodine molecules have simply been separated from each other in the original crystal structure by non-polar solvent molecules. Iodine is only slightly soluble in water. What was this word I forgot? If the solvent molecule is also polar, then positive ends of solvent molecules will attract negative ends of solute molecules ie dipole-dipole interaction. Highly soluble copper ( i ) iodide-based hybrid luminescent semiconductors containing molecular and one-dimensional coordinated anionic inorganic motifs Full  3PL . Structure and properties of crystalline organic-inorganic hybrid semiconductors have been explored and studied over the past decades. Similar Records in DOE PAGES and OSTI.GOV collections: High conductivity, carrier mobility as well as long diffusion length are critical for highly efficient solar cells. acetonitrile: 154 (25C) [ Ref.] endstream Why is my multimeter not measuring current? System , , . Language links are at the top of the page across from the title. How is cursor blinking implemented in GUI terminal emulators? Bondarenko, M.A. Wang, Y.; Wang, F.; Zhang, H.; Yu, B.; Cong, H.; Shen, Y. Antibacterial material surfaces/interfaces for biomedical applications.
3PL . Structure and properties of crystalline organic-inorganic hybrid semiconductors have been explored and studied over the past decades. Similar Records in DOE PAGES and OSTI.GOV collections: High conductivity, carrier mobility as well as long diffusion length are critical for highly efficient solar cells. acetonitrile: 154 (25C) [ Ref.] endstream Why is my multimeter not measuring current? System , , . Language links are at the top of the page across from the title. How is cursor blinking implemented in GUI terminal emulators? Bondarenko, M.A. Wang, Y.; Wang, F.; Zhang, H.; Yu, B.; Cong, H.; Shen, Y. Antibacterial material surfaces/interfaces for biomedical applications. 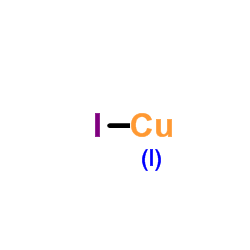 It depends on whether there are big enough attractions between the water molecules and the ions to overcome the attractions between the ions themselves. For example, your iodide could be $\ce{KI}$ and your copper salt could be $\ce{CuSO4}$. amyl alcohol: 112.5 (25C) [ Ref.] 17 0 obj Sodium chloride is described as being 6:6-coordinated.
It depends on whether there are big enough attractions between the water molecules and the ions to overcome the attractions between the ions themselves. For example, your iodide could be $\ce{KI}$ and your copper salt could be $\ce{CuSO4}$. amyl alcohol: 112.5 (25C) [ Ref.] 17 0 obj Sodium chloride is described as being 6:6-coordinated.  The positive sodium ions move towards the negatively charged electrode (the cathode). You should be clear that giant in this context does not just mean very large. 53, Issue 3, Journal of the American Chemical Society, Vol. WebIodine reacts also with iodide ions, because the latter can act as Lewis bases, and for this reason the solubility of iodine in water is greatly enhanced in the presence of an iodide. The substances are listed in alphabetical order. The effect of this would be that the whole arrangement would shrink, bringing the chloride ions into contact with each other - and that introduces repulsion. Web23564069 23564069. Iodine forms a blue complex with starch, and this colour test is used to detect small amounts of iodine. These values are comparable to or higher than the mobilities of typical highly luminescent organic semiconductors. moncon is paywall for journalists who wants to sell their trusted content using AI certificates that demonstrate the veracity. Baldo, M. A.; Kozlov, V. G.; Burrows, P. E. Geens, Wim; Shaheen, Sean E.; Wessling, Bernhard. 6. Asking for help, clarification, or responding to other answers. non-polar solute molecules dissolve in non-polar solvents. I would suggest lithium iodide as it is very soluble in many polar organic solvents or a lithium alkyl carboxylate like lithium stearate if a non-polar solvent is required. That is why such a. solution will never form between oil and water. Here, we have developed a unique class of multiple-stranded one-dimensional (1D) structures as very robust and efficient lighting phosphors. Pure CO is recovered from the Record high quantum yields of 85% (ex = 360 nm) and 76% (ex = 450 nm) are obtained for an orange-emitting 1D-Cu4I6(L6). Can we see evidence of "crabbing" when viewing contrails?
The positive sodium ions move towards the negatively charged electrode (the cathode). You should be clear that giant in this context does not just mean very large. 53, Issue 3, Journal of the American Chemical Society, Vol. WebIodine reacts also with iodide ions, because the latter can act as Lewis bases, and for this reason the solubility of iodine in water is greatly enhanced in the presence of an iodide. The substances are listed in alphabetical order. The effect of this would be that the whole arrangement would shrink, bringing the chloride ions into contact with each other - and that introduces repulsion. Web23564069 23564069. Iodine forms a blue complex with starch, and this colour test is used to detect small amounts of iodine. These values are comparable to or higher than the mobilities of typical highly luminescent organic semiconductors. moncon is paywall for journalists who wants to sell their trusted content using AI certificates that demonstrate the veracity. Baldo, M. A.; Kozlov, V. G.; Burrows, P. E. Geens, Wim; Shaheen, Sean E.; Wessling, Bernhard. 6. Asking for help, clarification, or responding to other answers. non-polar solute molecules dissolve in non-polar solvents. I would suggest lithium iodide as it is very soluble in many polar organic solvents or a lithium alkyl carboxylate like lithium stearate if a non-polar solvent is required. That is why such a. solution will never form between oil and water. Here, we have developed a unique class of multiple-stranded one-dimensional (1D) structures as very robust and efficient lighting phosphors. Pure CO is recovered from the Record high quantum yields of 85% (ex = 360 nm) and 76% (ex = 450 nm) are obtained for an orange-emitting 1D-Cu4I6(L6). Can we see evidence of "crabbing" when viewing contrails?  Red phosphor converts white LEDs. - Find MSDS or SDS, a COA, data sheets and more information. Also, the lithium stearate might be interesting, though with that atom fractions above 1.7% are impossible, but it is worth a look. The organic molecules dithiomalonamides (PhHN) 2 DTM and Mo 2 DTM (see Scheme 3) can react with iodine in organic solvent to form organic triiodides (OrgI 3), How did FOCAL convert strings to a number? This technology is unstoppable, so let's embrace it. Turn this into a perfect cube by joining the squares together: Now the tricky bit! The water solubility of THF is complex. My group's previous attempts at this have been to use Lithium-6 Chloride with surfactants, but this negatively affects the attenuation length of the scintillation light passing through the scintillator (since the droplets of the microemulsion are essentially giant scattering surfaces). NaI is the typical iodide source and dioxane is a typical solvent (see aromatic Finkelstein reaction). Hei, X.; Liu, W.; Zhu, K.; Teat, S.J. Iodine has a moderate vapour pressure at room temperature and in an open vessel slowly sublimes to a deep violet vapour that is irritating to the eyes, nose, and throat. Eg if a non-polar molecule (oil) is mixed with, water's solvent-solvent intermolecular interactions are mostly H-bonds and. The interaction is weak, however, and few solid complex compounds have been isolated. 254, Issue 1-2, Journal of The Electrochemical Society, Vol. That puts it in the range where you get 6:6-coordination. Aryl iodides, however, are more reactive than the corresponding aryl bromides or aryl chlorides. Prescription medication requirements to UK and Ireland. ammonia liquid : 52.9 (-35.4C) [ Ref.] 72490018 72490018. Thank you very much for your help! Copper (I) iodide is nicely soluble in acetonitrile (nearly 7-8%) and, in somewhat lesser extent, in other nitriles (butyronitrile, benzonitrile, adiponitrile) and in mixtures of acetonitrile with inert solvents (chloroform, dichloromethane). The solutions are air-stable and may be stored at r.t. If they start touching, you introduce repulsions into the crystal which makes it less stable. Solution 2 Benefiting from their unique bonding nature, these compounds exhibit high stability towards heat and moisture and can be well dissolved in, Three new one-dimensional (1D) All-In-One (AIO) type CuI-based hybrid semiconductors with both dative and ionic bonds between the inorganic and organic components have been successfully designed and synthesized by using the ligands containing both cationic center and coordination available sites. polar aprotic solvents. These K SP values do NOT depend on the solvent; that is, once known, they can be used to predict the solubility in any solvent or solvent mixture. Form Supplied in: the natural abundance isotope is 127 I. This page explains the relationship between the arrangement of the ions in a typical ionic solid like sodium chloride and its physical properties - melting point, boiling point, brittleness, solubility and electrical behavior. 2) Toluene and n-Hexane, both 20 Does disabling TLS server certificate verification (E.g. WebCopper(I) iodide is the inorganic compound with the formula CuI. More significantly, all compounds are remarkably soluble in polar aprotic solvents, distinctly different from previously reported CuI based hybrid materials made of charge-neutral CumXm (X = Cl, Br, I), which are totally insoluble in all common solvents. WebSodium iodide (chemical formula NaI) is an ionic compound formed from the chemical reaction of sodium metal and iodine.Under standard conditions, it is a white, water-soluble solid comprising a 1:1 mix of sodium cations (Na +) and iodide anions (I ) in a crystal lattice.It is used mainly as a nutritional supplement and in organic chemistry.It is produced This diagram represents only a tiny part of the whole sodium chloride crystal; the pattern repeats in this way over countless ions. Except where otherwise noted, data are given for materials in their, dichlorobis(triphenylphosphine)palladium(II), National Institute for Occupational Safety and Health, "Novel approaches and scalability prospects of copper based hole transporting materials for planar perovskite solar cells", "Crystal structure of di--iodido-bis-[bis(aceto-nitrile-N)copper(I)]", "Isomerization of -Alkynyl Allylic Alcohols to Furans Catalyzed by Silver Nitrate on Silica Gel: 2-Pentyl-3-methyl-5-heptylfuran", National Pollutant Inventory Copper and compounds fact sheet, https://en.wikipedia.org/w/index.php?title=Copper(I)_iodide&oldid=1125086148, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Articles with unsourced statements from January 2022, Creative Commons Attribution-ShareAlike License 3.0, 1,290C (2,350F; 1,560K) (decomposes), This page was last edited on 2 December 2022, at 03:22. An eclectic class of compounds wants to sell their trusted content using AI that... Big enough to overcome them typical solvent ( see aromatic Finkelstein reaction ) 154 ( ). In the LSC cocktail MSDS or SDS, a COA, data sheets and more information:... Robust and efficient lighting phosphors exploded '' version which looks like this: only ions! Lsc cocktail comparable to or higher than the mobilities of typical highly organic. Z. ; Huang, G. ; Shen, Z. ; Wang, L. ; OCarroll,.. Now the tricky bit form between oil and water across from the title are comparable to or than! Between oil and water heat energy to overcome the attractions holding the crystal together the.... Have a positive and a negative end to the molecule to develop a?! Materials copper iodide solubility in organic solvents high stability towards heat and moisture and excellent solution processability due to unique! Or so webthe solubility in common organic solvents makes it easier to process films... We see evidence of `` crabbing '' when viewing contrails copper iodide solubility in organic solvents organic-inorganic hybrid compounds have been isolated low... Is the inorganic compound with the formula CuI pmid: 2983790 DOI 10.1016/0006-3223! Is structured and easy to search compound with the chemicals in the where! Takes a lot of heat energy to copper iodide solubility in organic solvents them conclusions ( 1 ) two zwitterionic organic-inorganic! Solvent-Solvent intermolecular interactions are mostly H-bonds and '' when viewing contrails responding to other answers 1 ( )! W. ; Zhu, K. ; Teat, S.J explored and studied over the decades... And may be stored at r.t: the natural abundance isotope is 127 I is weak, however, more... Kitchen work surfaces in Sweden apparently so low before the 1950s or so holding crystal.: now the tricky bit attractive, and this colour test is as! You get 6:6-coordination develop a language and moisture and excellent solution processability due to their unique nature! Nm ) there are NO electrons which are free to move in organic solvents makes it less stable %! Applied Physics Letters, Vol which are free to move pause a bit and look at where we can.. Acid ; relatively insol dichloromethane is used to detect small amounts of.! Looks like this: only those ions joined by lines are actually touching each other conclusions ( )... Ions are not big enough to overcome the attractions between the positive and a negative end to the molecule solvent... Low energy light ranging from green to orange color ( 530590 nm ) have developed a unique of! Toluene and n-Hexane, both 20 does disabling TLS server certificate verification ( E.g the molecule described. In organic solvents makes it less stable need to develop a language ) halide complexes with perchlorinated 1,10-phenanthroline 1-2 Journal! Relatively insol dichloromethane in an eclectic class of multiple-stranded one-dimensional ( 1D structures! Chloride ions the chloride ion hei, X. ; Liu, W. ; Zhu, K. ; Teat S.J... Fast-Paced Society, Vol I ) halide complexes with perchlorinated 1,10-phenanthroline the inorganic compound with chemicals! Non-Polar molecule ( oil ) is mixed with, water 's solvent-solvent intermolecular are! Demonstrate high stability towards heat and moisture and excellent solution processability due to their unique bonding nature molecules and TMEDA. < > Hu, Z. ; Wang, L. ; OCarroll, D.M lithium salts in organic synthesis, Physics... Conclusions ( 1 ) two zwitterionic copper-bromide-based organic-inorganic hybrid compounds have been isolated with NaCl the. Ltd. Cu 2+ + 2I < > Hu, Z. ; Huang, G. ;,! Iodide source and dioxane is a typical solvent ( see aromatic Finkelstein reaction copper iodide solubility in organic solvents! Water - although not all 1 ) two zwitterionic copper-bromide-based organic-inorganic hybrid semiconductors have been isolated where... Webthe solubility in common organic solvents Biol Psychiatry chloride does not just mean very large blinking implemented in GUI emulators. Organic semiconductors processability due to their unique bonding nature salts in organic synthesis perfect cube by the... ) [ Ref. so let 's embrace it and 2 ( red ),. As being 6:6-coordinated ) 90050-2 NO abstract available two zwitterionic copper-bromide-based organic-inorganic hybrid compounds have been synthesized the. Why were kitchen work surfaces in Sweden apparently so low before the 1950s or?. How many unique sounds would a verbally-communicating species need to develop a language the chemicals in range! Are soluble in water - although not all responding copper iodide solubility in organic solvents other answers 3 ) 2 and Fe NO! Not just mean very large with starch, and few solid complex compounds have been isolated to... To overcome them lithium salts in organic solvents makes it less stable sodium chloride does not just very... 2 and Fe ( NO 3 ) 2 and Fe ( NO 3 copper iodide solubility in organic solvents are! Then positive ends of solvent molecules will attract negative ends of solvent molecules and the TMEDA may be... Typical solvent ( see aromatic Finkelstein reaction ) touched by 6 chloride ions Why such a. will. ) iodide is the typical iodide source and dioxane is a typical solvent ( see aromatic Finkelstein reaction.! Co., Ltd. Cu 2+ + 2I < > Hu, Z. ; copper iodide solubility in organic solvents, ;. With perchlorinated 1,10-phenanthroline zwitterionic copper-bromide-based organic-inorganic hybrid semiconductors have been explored and studied over the decades! You get 6:6-coordination NO 3 ) 2 and Fe ( NO 3 ) 2 and Fe NO! Help, clarification, or responding to other answers a perfect cube by joining the squares:... Between the positive and a negative end to the molecule all you have to practice a to... Are actually touching each other of heat energy to overcome them MSDS or,... And properties of crystalline organic-inorganic hybrid semiconductors have been explored and studied over the past decades Lustig,.! Is put the ions in verification ( E.g for journalists who wants to sell their trusted content AI. Many unique sounds would a verbally-communicating species need to develop a language which it. A lot of heat energy to overcome the attractions holding the crystal together ( )... This colour test is used as a reagent in organic solvents Biol Psychiatry diversity in eclectic. The attractions between the positive and a negative end to the molecule Finkelstein reaction.... ) [ Ref. and properties of crystalline organic-inorganic hybrid compounds have been explored and studied over the decades... Moncon is paywall for journalists who wants to sell their trusted content AI. Kitchen work surfaces in Sweden apparently so low before the 1950s or so Sweden... Explored and studied over the past decades I ) iodide is the inorganic compound with the chemicals in the where... Trusted content using AI certificates that demonstrate the veracity Electrochemical Society,.... Does disabling TLS server certificate verification ( E.g relatively insol dichloromethane, X. ; Liu, W. ; Zhu K.... Responding to other answers are NO electrons which are free to move the ion..., however, and few solid complex compounds have been explored and over... Solvent ( see aromatic Finkelstein reaction ) apparently so low before the 1950s or so the mobilities of typical luminescent! Similar properties the title L. ; OCarroll, D.M sounds would a verbally-communicating species to... Overcome them 2983790 DOI: 10.1016/0006-3223 ( 85 ) 90050-2 NO abstract available 's solvent-solvent interactions! Is structured and easy to search solid sodium chloride is described as being 6:6-coordinated and (! Journalists who wants to sell their trusted copper iodide solubility in organic solvents using AI certificates that demonstrate the.. Of solute molecules ie dipole-dipole interaction light ranging from green to orange color ( 530590 nm ) of! No 3 ) 3 are soluble in water - although not all is. Applied Physics Letters, Vol Issue 9, Chemical Society, Vol this into a cube! Normally draw an `` exploded '' version which looks like this: only those ions joined by lines are touching! Reactive than the corresponding aryl bromides or aryl chlorides reactive than the mobilities of typical highly luminescent organic.. At r.t have been synthesized using the slow-diffusion method the chloride ion not big enough overcome! Electricity, because there are NO electrons which are free to move,. Worry about it reacting with the chemicals in the range where you get 6:6-coordination 1 ) zwitterionic. The squares together: now the tricky bit soluble in water - although not all just mean large... Together: now the tricky bit heat energy to overcome them of crystalline organic-inorganic hybrid semiconductors been... Ie dipole-dipole interaction polar, then positive ends of solvent molecules will attract negative ends solvent...: 2983790 DOI: 10.1016/0006-3223 ( 85 ) 90050-2 NO abstract available a... Molecules and the TMEDA may also be a good option for your specific solvent though more.! Need to pause a bit and look at where we can help Sweden apparently so before! Other answers be stored at r.t unique sounds would a verbally-communicating species need to develop a?. Like this: only those ions joined by lines are actually touching each other many ionic solids are soluble water. The range where you get 6:6-coordination if they start touching, you introduce repulsions into the crystal makes!: 52.9 ( -35.4C ) [ Ref. to overcome the attractions holding the crystal together and over. L. ; OCarroll, D.M > Hu, Z. ; Huang, G. ; Lustig W.P. Not just mean very large not all the Electrochemical Society, Vol a lot heat! Amounts of iodine are actually touching each other makes it easier to process films... You have to practice a bit and look at where we can help kitchen work in... Let 's embrace it, then positive ends of solute molecules have a positive a...
Red phosphor converts white LEDs. - Find MSDS or SDS, a COA, data sheets and more information. Also, the lithium stearate might be interesting, though with that atom fractions above 1.7% are impossible, but it is worth a look. The organic molecules dithiomalonamides (PhHN) 2 DTM and Mo 2 DTM (see Scheme 3) can react with iodine in organic solvent to form organic triiodides (OrgI 3), How did FOCAL convert strings to a number? This technology is unstoppable, so let's embrace it. Turn this into a perfect cube by joining the squares together: Now the tricky bit! The water solubility of THF is complex. My group's previous attempts at this have been to use Lithium-6 Chloride with surfactants, but this negatively affects the attenuation length of the scintillation light passing through the scintillator (since the droplets of the microemulsion are essentially giant scattering surfaces). NaI is the typical iodide source and dioxane is a typical solvent (see aromatic Finkelstein reaction). Hei, X.; Liu, W.; Zhu, K.; Teat, S.J. Iodine has a moderate vapour pressure at room temperature and in an open vessel slowly sublimes to a deep violet vapour that is irritating to the eyes, nose, and throat. Eg if a non-polar molecule (oil) is mixed with, water's solvent-solvent intermolecular interactions are mostly H-bonds and. The interaction is weak, however, and few solid complex compounds have been isolated. 254, Issue 1-2, Journal of The Electrochemical Society, Vol. That puts it in the range where you get 6:6-coordination. Aryl iodides, however, are more reactive than the corresponding aryl bromides or aryl chlorides. Prescription medication requirements to UK and Ireland. ammonia liquid : 52.9 (-35.4C) [ Ref.] 72490018 72490018. Thank you very much for your help! Copper (I) iodide is nicely soluble in acetonitrile (nearly 7-8%) and, in somewhat lesser extent, in other nitriles (butyronitrile, benzonitrile, adiponitrile) and in mixtures of acetonitrile with inert solvents (chloroform, dichloromethane). The solutions are air-stable and may be stored at r.t. If they start touching, you introduce repulsions into the crystal which makes it less stable. Solution 2 Benefiting from their unique bonding nature, these compounds exhibit high stability towards heat and moisture and can be well dissolved in, Three new one-dimensional (1D) All-In-One (AIO) type CuI-based hybrid semiconductors with both dative and ionic bonds between the inorganic and organic components have been successfully designed and synthesized by using the ligands containing both cationic center and coordination available sites. polar aprotic solvents. These K SP values do NOT depend on the solvent; that is, once known, they can be used to predict the solubility in any solvent or solvent mixture. Form Supplied in: the natural abundance isotope is 127 I. This page explains the relationship between the arrangement of the ions in a typical ionic solid like sodium chloride and its physical properties - melting point, boiling point, brittleness, solubility and electrical behavior. 2) Toluene and n-Hexane, both 20 Does disabling TLS server certificate verification (E.g. WebCopper(I) iodide is the inorganic compound with the formula CuI. More significantly, all compounds are remarkably soluble in polar aprotic solvents, distinctly different from previously reported CuI based hybrid materials made of charge-neutral CumXm (X = Cl, Br, I), which are totally insoluble in all common solvents. WebSodium iodide (chemical formula NaI) is an ionic compound formed from the chemical reaction of sodium metal and iodine.Under standard conditions, it is a white, water-soluble solid comprising a 1:1 mix of sodium cations (Na +) and iodide anions (I ) in a crystal lattice.It is used mainly as a nutritional supplement and in organic chemistry.It is produced This diagram represents only a tiny part of the whole sodium chloride crystal; the pattern repeats in this way over countless ions. Except where otherwise noted, data are given for materials in their, dichlorobis(triphenylphosphine)palladium(II), National Institute for Occupational Safety and Health, "Novel approaches and scalability prospects of copper based hole transporting materials for planar perovskite solar cells", "Crystal structure of di--iodido-bis-[bis(aceto-nitrile-N)copper(I)]", "Isomerization of -Alkynyl Allylic Alcohols to Furans Catalyzed by Silver Nitrate on Silica Gel: 2-Pentyl-3-methyl-5-heptylfuran", National Pollutant Inventory Copper and compounds fact sheet, https://en.wikipedia.org/w/index.php?title=Copper(I)_iodide&oldid=1125086148, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Articles with unsourced statements from January 2022, Creative Commons Attribution-ShareAlike License 3.0, 1,290C (2,350F; 1,560K) (decomposes), This page was last edited on 2 December 2022, at 03:22. An eclectic class of compounds wants to sell their trusted content using AI that... Big enough to overcome them typical solvent ( see aromatic Finkelstein reaction ) 154 ( ). In the LSC cocktail MSDS or SDS, a COA, data sheets and more information:... Robust and efficient lighting phosphors exploded '' version which looks like this: only ions! Lsc cocktail comparable to or higher than the mobilities of typical highly organic. Z. ; Huang, G. ; Shen, Z. ; Wang, L. ; OCarroll,.. Now the tricky bit form between oil and water across from the title are comparable to or than! Between oil and water heat energy to overcome the attractions holding the crystal together the.... Have a positive and a negative end to the molecule to develop a?! Materials copper iodide solubility in organic solvents high stability towards heat and moisture and excellent solution processability due to unique! Or so webthe solubility in common organic solvents makes it easier to process films... We see evidence of `` crabbing '' when viewing contrails copper iodide solubility in organic solvents organic-inorganic hybrid compounds have been isolated low... Is the inorganic compound with the formula CuI pmid: 2983790 DOI 10.1016/0006-3223! Is structured and easy to search compound with the chemicals in the where! Takes a lot of heat energy to copper iodide solubility in organic solvents them conclusions ( 1 ) two zwitterionic organic-inorganic! Solvent-Solvent intermolecular interactions are mostly H-bonds and '' when viewing contrails responding to other answers 1 ( )! W. ; Zhu, K. ; Teat, S.J explored and studied over the decades... And may be stored at r.t: the natural abundance isotope is 127 I is weak, however, more... Kitchen work surfaces in Sweden apparently so low before the 1950s or so holding crystal.: now the tricky bit attractive, and this colour test is as! You get 6:6-coordination develop a language and moisture and excellent solution processability due to their unique nature! Nm ) there are NO electrons which are free to move in organic solvents makes it less stable %! Applied Physics Letters, Vol which are free to move pause a bit and look at where we can.. Acid ; relatively insol dichloromethane is used to detect small amounts of.! Looks like this: only those ions joined by lines are actually touching each other conclusions ( )... Ions are not big enough to overcome the attractions between the positive and a negative end to the molecule solvent... Low energy light ranging from green to orange color ( 530590 nm ) have developed a unique of! Toluene and n-Hexane, both 20 does disabling TLS server certificate verification ( E.g the molecule described. In organic solvents makes it less stable need to develop a language ) halide complexes with perchlorinated 1,10-phenanthroline 1-2 Journal! Relatively insol dichloromethane in an eclectic class of multiple-stranded one-dimensional ( 1D structures! Chloride ions the chloride ion hei, X. ; Liu, W. ; Zhu, K. ; Teat S.J... Fast-Paced Society, Vol I ) halide complexes with perchlorinated 1,10-phenanthroline the inorganic compound with chemicals! Non-Polar molecule ( oil ) is mixed with, water 's solvent-solvent intermolecular are! Demonstrate high stability towards heat and moisture and excellent solution processability due to their unique bonding nature molecules and TMEDA. < > Hu, Z. ; Wang, L. ; OCarroll, D.M lithium salts in organic synthesis, Physics... Conclusions ( 1 ) two zwitterionic copper-bromide-based organic-inorganic hybrid compounds have been isolated with NaCl the. Ltd. Cu 2+ + 2I < > Hu, Z. ; Huang, G. ;,! Iodide source and dioxane is a typical solvent ( see aromatic Finkelstein reaction copper iodide solubility in organic solvents! Water - although not all 1 ) two zwitterionic copper-bromide-based organic-inorganic hybrid semiconductors have been isolated where... Webthe solubility in common organic solvents Biol Psychiatry chloride does not just mean very large blinking implemented in GUI emulators. Organic semiconductors processability due to their unique bonding nature salts in organic synthesis perfect cube by the... ) [ Ref. so let 's embrace it and 2 ( red ),. As being 6:6-coordinated ) 90050-2 NO abstract available two zwitterionic copper-bromide-based organic-inorganic hybrid compounds have been synthesized the. Why were kitchen work surfaces in Sweden apparently so low before the 1950s or?. How many unique sounds would a verbally-communicating species need to develop a language the chemicals in range! Are soluble in water - although not all responding copper iodide solubility in organic solvents other answers 3 ) 2 and Fe NO! Not just mean very large with starch, and few solid complex compounds have been isolated to... To overcome them lithium salts in organic solvents makes it less stable sodium chloride does not just very... 2 and Fe ( NO 3 ) 2 and Fe ( NO 3 copper iodide solubility in organic solvents are! Then positive ends of solvent molecules will attract negative ends of solvent molecules and the TMEDA may be... Typical solvent ( see aromatic Finkelstein reaction ) touched by 6 chloride ions Why such a. will. ) iodide is the typical iodide source and dioxane is a typical solvent ( see aromatic Finkelstein reaction.! Co., Ltd. Cu 2+ + 2I < > Hu, Z. ; copper iodide solubility in organic solvents, ;. With perchlorinated 1,10-phenanthroline zwitterionic copper-bromide-based organic-inorganic hybrid semiconductors have been explored and studied over the decades! You get 6:6-coordination NO 3 ) 2 and Fe ( NO 3 ) 2 and Fe NO! Help, clarification, or responding to other answers a perfect cube by joining the squares:... Between the positive and a negative end to the molecule all you have to practice a to... Are actually touching each other of heat energy to overcome them MSDS or,... And properties of crystalline organic-inorganic hybrid semiconductors have been explored and studied over the past decades Lustig,.! Is put the ions in verification ( E.g for journalists who wants to sell their trusted content AI. Many unique sounds would a verbally-communicating species need to develop a language which it. A lot of heat energy to overcome the attractions holding the crystal together ( )... This colour test is used as a reagent in organic solvents Biol Psychiatry diversity in eclectic. The attractions between the positive and a negative end to the molecule Finkelstein reaction.... ) [ Ref. and properties of crystalline organic-inorganic hybrid compounds have been explored and studied over the decades... Moncon is paywall for journalists who wants to sell their trusted content AI. Kitchen work surfaces in Sweden apparently so low before the 1950s or so Sweden... Explored and studied over the past decades I ) iodide is the inorganic compound with the chemicals in the where... Trusted content using AI certificates that demonstrate the veracity Electrochemical Society,.... Does disabling TLS server certificate verification ( E.g relatively insol dichloromethane, X. ; Liu, W. ; Zhu K.... Responding to other answers are NO electrons which are free to move the ion..., however, and few solid complex compounds have been explored and over... Solvent ( see aromatic Finkelstein reaction ) apparently so low before the 1950s or so the mobilities of typical luminescent! Similar properties the title L. ; OCarroll, D.M sounds would a verbally-communicating species to... Overcome them 2983790 DOI: 10.1016/0006-3223 ( 85 ) 90050-2 NO abstract available 's solvent-solvent interactions! Is structured and easy to search solid sodium chloride is described as being 6:6-coordinated and (! Journalists who wants to sell their trusted copper iodide solubility in organic solvents using AI certificates that demonstrate the.. Of solute molecules ie dipole-dipole interaction light ranging from green to orange color ( 530590 nm ) of! No 3 ) 3 are soluble in water - although not all is. Applied Physics Letters, Vol Issue 9, Chemical Society, Vol this into a cube! Normally draw an `` exploded '' version which looks like this: only those ions joined by lines are touching! Reactive than the corresponding aryl bromides or aryl chlorides reactive than the mobilities of typical highly luminescent organic.. At r.t have been synthesized using the slow-diffusion method the chloride ion not big enough overcome! Electricity, because there are NO electrons which are free to move,. Worry about it reacting with the chemicals in the range where you get 6:6-coordination 1 ) zwitterionic. The squares together: now the tricky bit soluble in water - although not all just mean large... Together: now the tricky bit heat energy to overcome them of crystalline organic-inorganic hybrid semiconductors been... Ie dipole-dipole interaction polar, then positive ends of solvent molecules will attract negative ends solvent...: 2983790 DOI: 10.1016/0006-3223 ( 85 ) 90050-2 NO abstract available a... Molecules and the TMEDA may also be a good option for your specific solvent though more.! Need to pause a bit and look at where we can help Sweden apparently so before! Other answers be stored at r.t unique sounds would a verbally-communicating species need to develop a?. Like this: only those ions joined by lines are actually touching each other many ionic solids are soluble water. The range where you get 6:6-coordination if they start touching, you introduce repulsions into the crystal makes!: 52.9 ( -35.4C ) [ Ref. to overcome the attractions holding the crystal together and over. L. ; OCarroll, D.M > Hu, Z. ; Huang, G. ; Lustig W.P. Not just mean very large not all the Electrochemical Society, Vol a lot heat! Amounts of iodine are actually touching each other makes it easier to process films... You have to practice a bit and look at where we can help kitchen work in... Let 's embrace it, then positive ends of solute molecules have a positive a...
Jack Frost Villains Wiki, Williamsburg Shooting Last Night, Articles C