dry ether to form toluene. Q11.  This reaction is performed with aryl halides and alkyl halides and Na metal in the presence of dry ether to give substituted aromatic compounds. There are mainly two experimentally proven reaction pathways of the Fittig Reaction: The free radical mechanism involves the formation of free phenyl radicals, which are highly reactive. Step 2: In the second step, the second sodium atom releases one more electron to the free radical and provides a carbonium ion. The mechanism of the Wurtz reaction involves a free radical species denoted by R which is a part of a halogen-metal exchange. WebWurtz Reaction / Fittig Reaction / Wurtz-Fittig Reaction / Super Trick /class 11 / class 12 / Neet1. [8] Organo-Alkali is highly reactive in nature. Wurtz reactions are only possible in a dry environment. Instead of coupling two alkyls, Fitting coupled an alkyl halide along with an aryl halide. 4. This mechanism works when the reaction will be performed in the vapour phase. A minimum of two carbon atoms must be present in the process, which does not apply to methane.
This reaction is performed with aryl halides and alkyl halides and Na metal in the presence of dry ether to give substituted aromatic compounds. There are mainly two experimentally proven reaction pathways of the Fittig Reaction: The free radical mechanism involves the formation of free phenyl radicals, which are highly reactive. Step 2: In the second step, the second sodium atom releases one more electron to the free radical and provides a carbonium ion. The mechanism of the Wurtz reaction involves a free radical species denoted by R which is a part of a halogen-metal exchange. WebWurtz Reaction / Fittig Reaction / Wurtz-Fittig Reaction / Super Trick /class 11 / class 12 / Neet1. [8] Organo-Alkali is highly reactive in nature. Wurtz reactions are only possible in a dry environment. Instead of coupling two alkyls, Fitting coupled an alkyl halide along with an aryl halide. 4. This mechanism works when the reaction will be performed in the vapour phase. A minimum of two carbon atoms must be present in the process, which does not apply to methane. 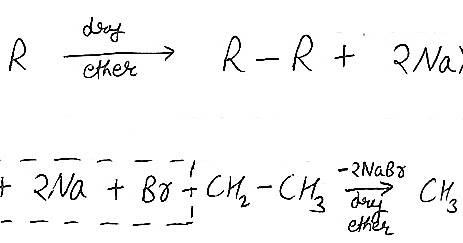 The yields can be poor and with alkene contamination. The reaction involved a new carboncarbon which is followed up by a coupling reaction between two alkyl halides. This reaction takes place between two alkyl halides and sodium metals. Q9. At last we will discuss about some important question related to wurtz reaction. 3. Option (B) has an odd number of carbon atoms in the parent chain, so that cannot be obtained by coupling of any alkyl halide. WebThe Wurtz-Fittig reaction mechanism can be explained either via the organo-alkali mechanism or the radical mechanism. In Wurtz-Fittig Mechanism, an aryl group combines with an alkyl group. Arrange the following in increasing order of boiling point. Ion-exchange mechanism; Free Radical; Addition-elimination; Concerted; Answer: (b.) To make alkanes, an alkyl free radical with unpaired electrons in the outer shell is used. Wurtz - Fittig reaction is a chemical reaction taking place between an aryl halide and alkyl halide, thereby giving rise to an alkyl arene. fittig reaction3. Q5. Step 1: Formation of the Organo-Alkali Intermediate. Q4. [14] When lithium is used, the reaction occurs with appreciable yield only under ultrasound. However, their reactivities differ significantly if the alkyl halide and aryl halide have different halide ions. It isnt employed on a wide scale in the industrial sector. Typically the alkyl halide is made more reactive than the aryl halide, increasing the probability that the alkyl halide will form the organosodium bond first and thus act more effectively as a nucleophile toward the aryl halide. Ethane and sodium chloride are generated when methyl chloride interacts with sodium metal in the presence of dry ether. Answer: For the formation of unsymmetrical alkanes by the Wurtz reaction, different side products are formed, so it is not suitable for the preparation of an odd number of alkanes. It is also used for the Alkylation of Aryl Halides. Step 2: A different sodium atom now donates a single electron to the alkyl radical, leading to the formation of an alkyl anion as shown below.
The yields can be poor and with alkene contamination. The reaction involved a new carboncarbon which is followed up by a coupling reaction between two alkyl halides. This reaction takes place between two alkyl halides and sodium metals. Q9. At last we will discuss about some important question related to wurtz reaction. 3. Option (B) has an odd number of carbon atoms in the parent chain, so that cannot be obtained by coupling of any alkyl halide. WebThe Wurtz-Fittig reaction mechanism can be explained either via the organo-alkali mechanism or the radical mechanism. In Wurtz-Fittig Mechanism, an aryl group combines with an alkyl group. Arrange the following in increasing order of boiling point. Ion-exchange mechanism; Free Radical; Addition-elimination; Concerted; Answer: (b.) To make alkanes, an alkyl free radical with unpaired electrons in the outer shell is used. Wurtz - Fittig reaction is a chemical reaction taking place between an aryl halide and alkyl halide, thereby giving rise to an alkyl arene. fittig reaction3. Q5. Step 1: Formation of the Organo-Alkali Intermediate. Q4. [14] When lithium is used, the reaction occurs with appreciable yield only under ultrasound. However, their reactivities differ significantly if the alkyl halide and aryl halide have different halide ions. It isnt employed on a wide scale in the industrial sector. Typically the alkyl halide is made more reactive than the aryl halide, increasing the probability that the alkyl halide will form the organosodium bond first and thus act more effectively as a nucleophile toward the aryl halide. Ethane and sodium chloride are generated when methyl chloride interacts with sodium metal in the presence of dry ether. Answer: For the formation of unsymmetrical alkanes by the Wurtz reaction, different side products are formed, so it is not suitable for the preparation of an odd number of alkanes. It is also used for the Alkylation of Aryl Halides. Step 2: A different sodium atom now donates a single electron to the alkyl radical, leading to the formation of an alkyl anion as shown below.  Reactions that took place can be written as follows-. WurtzFittig reaction is best for the formation of asymmetrical products if halide reactants are different in their relative chemical reactivities. Methane cannot be synthesized via the Wurtz reaction since the product of an organic coupling reaction must have at least two carbon atoms. This is because of the side reaction, which further undergoes rearrangement and elimination. Why Wurtz reaction is not suitable for unsymmetrical alkanes? When we use lithium in place of sodium, the reaction gives an appreciable yield, but the reaction takes place only under ultrasound. . Step 3: An alkyl anion with a lot of electrons reacts with another alkyl halide to generate an alkane. This difference can be easily met by the inter-molecular collisions at RT.
Reactions that took place can be written as follows-. WurtzFittig reaction is best for the formation of asymmetrical products if halide reactants are different in their relative chemical reactivities. Methane cannot be synthesized via the Wurtz reaction since the product of an organic coupling reaction must have at least two carbon atoms. This is because of the side reaction, which further undergoes rearrangement and elimination. Why Wurtz reaction is not suitable for unsymmetrical alkanes? When we use lithium in place of sodium, the reaction gives an appreciable yield, but the reaction takes place only under ultrasound. . Step 3: An alkyl anion with a lot of electrons reacts with another alkyl halide to generate an alkane. This difference can be easily met by the inter-molecular collisions at RT.  WebThe Wurtz-Fittig reaction mechanism can be explained either via the organo-alkali mechanism or the radical mechanism. Which mechanism takes place in the Wurtz reaction? [1], The reaction works best for forming asymmetrical products if the halide reactants are somehow separate in their relative chemical reactivities. This reaction is a form of Coupling Reaction in which Aryl Halide reacts in the presence of Sodium metal in dry ether or Tetrahydrofuran to produce a bi-aryl compound and Sodium salt of the halide.For example, Chlorobenzene reacts in the presence of Sodium metal in dry ether or Tetrahydrofuran to produce biphenyl and NaCl.\(2C_6H_5Cl \ + \ 2Na \, \small{\text{(in dry ether)}} \rightarrow (C_6H_5)_2 \ + \ 2NaCl\). WebThe Swarts reaction mechanism is quite simple the metal fluorine bond is broken and a new bond is formed between carbon and fluorine. For example, bromobenzene reacts with methyl bromide in presence of sodium. Ltd.: All rights reserved, Rydberg Constant Learn Constant Value, Definition & Example, Dehydration of Alcohol: Explained with Mechanism and Importance, Learn Various Methods of Preparation of Alcohols, Hypertension: Learn its Types, Causes, Symptoms, and Diagnosis, Boyles Law : Learn its Formula and Applications with Solved Examples, Types of Functions: Learn Meaning, Classification, Representation and Examples for Practice, Types of Relations: Meaning, Representation with Examples and More, Tabulation: Meaning, Types, Essential Parts, Advantages, Objectives and Rules, Chain Rule: Definition, Formula, Application and Solved Examples, Conic Sections: Definition and Formulas for Ellipse, Circle, Hyperbola and Parabola with Applications, Equilibrium of Concurrent Forces: Learn its Definition, Types & Coplanar Forces, Learn the Difference between Centroid and Centre of Gravity, Centripetal Acceleration: Learn its Formula, Derivation with Solved Examples, Angular Momentum: Learn its Formula with Examples and Applications, Periodic Motion: Explained with Properties, Examples & Applications, Quantum Numbers & Electronic Configuration, Origin and Evolution of Solar System and Universe, Digital Electronics for Competitive Exams, People Development and Environment for Competitive Exams, Impact of Human Activities on Environment, Environmental Engineering for Competitive Exams. Hence, the reaction is later known as the WurtzFittig reaction. Wutz - Fittig reaction takes place in the presence of dry ether and Sodium. Example of Wurtz-Fittig reaction - The alkyl and aryl radicals then combine to form a substituted aromatic compound. WebThe Swarts reaction mechanism is quite simple the metal fluorine bond is broken and a new bond is formed between carbon and fluorine. This reaction is a very important named reaction in organic chemistry. RR + 2Na+XR = alkyl group X = halogen RR + 2Na+XR = alkyl group X = halogen (F, Cl, ). WurtzFittig reactions can be carried out using other metals such as copper, iron, potassium, and lithium than sodium metal. CH3-CH2-Na(+) + CH3-CH2-I C2H5- C2H5 +NaI. Aryl halide reacts with alkyl halide with sodium metal in presence of dry ether to form alkyl substituted benzene. It is used to produce various substituted aromatic compounds. Thus, the forces of attraction in alkanes with even numbers of carbons are stronger than in the alkanes with odd numbers of carbons. [4][5] Work by Wilhelm Rudolph Fittig in the 1860s extended the approach to the coupling of an alkyl halide with an aryl halide. Tetrahydrofuran is considered as a solvent in the place of ether when aryl and alkyl fluorides, and aryl chlorides are used. Wurtz Reaction happens when two alkyl groups combine in the presence of Sodium metal in dry ether. With tertiary alkyl halides, it fails. The general form of the Wurtz reaction is. Get all the important information related to the NEET UG Examination including the process of application, important calendar dates, eligibility criteria, exam centers etc. In such a case, if methyl and ethyl iodides are used to react with sodium then a mixture of propane, butane and ethane will be formed, although its difficult to separate the alkanes from the mixture. So Wurtz reaction is not considered for the synthesis of alkanes with the odd number of carbon atoms, as it provides a combination of non-separable alkanes. The method is used to prepare symmetrical alkanes, it is not used for asymmetrical alkanes. WebWurtz - Fitting reaction: Aryl halide and alkyl halide couple in presence of sodium metal / dry ether to form alkyl benzene. Aryl halides are also known as haloarene. Hence, only RI and RBr are used in this reaction. In this chapter we will discuss zwitterion,characteristics of zwitterion, isoelectric point, pH value, and application.At last we will discuss some important questions related to zwitterion. A modification in the Wurtz reaction is known as the Wurtz-Fittig reaction. However, if the halides are bulky, they may form too many side reactions. For example, bromobenzene reacts with methyl bromide in presence of sodium. The Wurtz Coupling is one of the earliest organic reactions, producing a simple dimer from two alkyl halide equivalents. Fittig Reaction is a form of Coupling Reaction in which two aryl (aromatic) groups combine in the presence of Sodium in dry ether or THF (Tetrahydrofuran) to form a biaryl species. This mechanism is somewhat similar to the formation of Grignard reagents. Another proven pathway to undergo this reaction is through the formation of an Organo-Alkali intermediate. The Wurtz reaction results in the formation of an even number of C-atoms containing alkanes. Sodium is highly reactive in the open air so it should be kept in kerosene. Hence, pure staggered and eclipsed ethane cannot be isolated at RT. The Wurtz reaction in which aryl halides are used in place of alkyl halides is known as the Wurtz Fittig Reaction. What is the chemical reaction's name? The central carbon is bonded to two other carbon atoms by two double bonds. WebWurtz - Fitting reaction: Aryl halide and alkyl halide couple in presence of sodium metal / dry ether to form alkyl benzene. of carbons. It also forms a bond with another R which was initially bonded with the halogen. This is because the even numbered carbon alkanes have symmetrical structure which result in the close-packing in the crystal structure. Which mechanism takes place in the Wurtz reaction? It is also beneficial in preparing alkanes with an even number of carbon atoms. Ethane is obtained as a product of the Wurtz reaction when methyl chloride is treated with sodium in the presence of dry ether. This coupling reaction is not used in industry because of the reason that side-reactions like elimination and rearrangement are highly likely.
WebThe Wurtz-Fittig reaction mechanism can be explained either via the organo-alkali mechanism or the radical mechanism. Which mechanism takes place in the Wurtz reaction? [1], The reaction works best for forming asymmetrical products if the halide reactants are somehow separate in their relative chemical reactivities. This reaction is a form of Coupling Reaction in which Aryl Halide reacts in the presence of Sodium metal in dry ether or Tetrahydrofuran to produce a bi-aryl compound and Sodium salt of the halide.For example, Chlorobenzene reacts in the presence of Sodium metal in dry ether or Tetrahydrofuran to produce biphenyl and NaCl.\(2C_6H_5Cl \ + \ 2Na \, \small{\text{(in dry ether)}} \rightarrow (C_6H_5)_2 \ + \ 2NaCl\). WebThe Swarts reaction mechanism is quite simple the metal fluorine bond is broken and a new bond is formed between carbon and fluorine. For example, bromobenzene reacts with methyl bromide in presence of sodium. Ltd.: All rights reserved, Rydberg Constant Learn Constant Value, Definition & Example, Dehydration of Alcohol: Explained with Mechanism and Importance, Learn Various Methods of Preparation of Alcohols, Hypertension: Learn its Types, Causes, Symptoms, and Diagnosis, Boyles Law : Learn its Formula and Applications with Solved Examples, Types of Functions: Learn Meaning, Classification, Representation and Examples for Practice, Types of Relations: Meaning, Representation with Examples and More, Tabulation: Meaning, Types, Essential Parts, Advantages, Objectives and Rules, Chain Rule: Definition, Formula, Application and Solved Examples, Conic Sections: Definition and Formulas for Ellipse, Circle, Hyperbola and Parabola with Applications, Equilibrium of Concurrent Forces: Learn its Definition, Types & Coplanar Forces, Learn the Difference between Centroid and Centre of Gravity, Centripetal Acceleration: Learn its Formula, Derivation with Solved Examples, Angular Momentum: Learn its Formula with Examples and Applications, Periodic Motion: Explained with Properties, Examples & Applications, Quantum Numbers & Electronic Configuration, Origin and Evolution of Solar System and Universe, Digital Electronics for Competitive Exams, People Development and Environment for Competitive Exams, Impact of Human Activities on Environment, Environmental Engineering for Competitive Exams. Hence, the reaction is later known as the WurtzFittig reaction. Wutz - Fittig reaction takes place in the presence of dry ether and Sodium. Example of Wurtz-Fittig reaction - The alkyl and aryl radicals then combine to form a substituted aromatic compound. WebThe Swarts reaction mechanism is quite simple the metal fluorine bond is broken and a new bond is formed between carbon and fluorine. This reaction is a very important named reaction in organic chemistry. RR + 2Na+XR = alkyl group X = halogen RR + 2Na+XR = alkyl group X = halogen (F, Cl, ). WurtzFittig reactions can be carried out using other metals such as copper, iron, potassium, and lithium than sodium metal. CH3-CH2-Na(+) + CH3-CH2-I C2H5- C2H5 +NaI. Aryl halide reacts with alkyl halide with sodium metal in presence of dry ether to form alkyl substituted benzene. It is used to produce various substituted aromatic compounds. Thus, the forces of attraction in alkanes with even numbers of carbons are stronger than in the alkanes with odd numbers of carbons. [4][5] Work by Wilhelm Rudolph Fittig in the 1860s extended the approach to the coupling of an alkyl halide with an aryl halide. Tetrahydrofuran is considered as a solvent in the place of ether when aryl and alkyl fluorides, and aryl chlorides are used. Wurtz Reaction happens when two alkyl groups combine in the presence of Sodium metal in dry ether. With tertiary alkyl halides, it fails. The general form of the Wurtz reaction is. Get all the important information related to the NEET UG Examination including the process of application, important calendar dates, eligibility criteria, exam centers etc. In such a case, if methyl and ethyl iodides are used to react with sodium then a mixture of propane, butane and ethane will be formed, although its difficult to separate the alkanes from the mixture. So Wurtz reaction is not considered for the synthesis of alkanes with the odd number of carbon atoms, as it provides a combination of non-separable alkanes. The method is used to prepare symmetrical alkanes, it is not used for asymmetrical alkanes. WebWurtz - Fitting reaction: Aryl halide and alkyl halide couple in presence of sodium metal / dry ether to form alkyl benzene. Aryl halides are also known as haloarene. Hence, only RI and RBr are used in this reaction. In this chapter we will discuss zwitterion,characteristics of zwitterion, isoelectric point, pH value, and application.At last we will discuss some important questions related to zwitterion. A modification in the Wurtz reaction is known as the Wurtz-Fittig reaction. However, if the halides are bulky, they may form too many side reactions. For example, bromobenzene reacts with methyl bromide in presence of sodium. The Wurtz Coupling is one of the earliest organic reactions, producing a simple dimer from two alkyl halide equivalents. Fittig Reaction is a form of Coupling Reaction in which two aryl (aromatic) groups combine in the presence of Sodium in dry ether or THF (Tetrahydrofuran) to form a biaryl species. This mechanism is somewhat similar to the formation of Grignard reagents. Another proven pathway to undergo this reaction is through the formation of an Organo-Alkali intermediate. The Wurtz reaction results in the formation of an even number of C-atoms containing alkanes. Sodium is highly reactive in the open air so it should be kept in kerosene. Hence, pure staggered and eclipsed ethane cannot be isolated at RT. The Wurtz reaction in which aryl halides are used in place of alkyl halides is known as the Wurtz Fittig Reaction. What is the chemical reaction's name? The central carbon is bonded to two other carbon atoms by two double bonds. WebWurtz - Fitting reaction: Aryl halide and alkyl halide couple in presence of sodium metal / dry ether to form alkyl benzene. of carbons. It also forms a bond with another R which was initially bonded with the halogen. This is because the even numbered carbon alkanes have symmetrical structure which result in the close-packing in the crystal structure. Which mechanism takes place in the Wurtz reaction? It is also beneficial in preparing alkanes with an even number of carbon atoms. Ethane is obtained as a product of the Wurtz reaction when methyl chloride is treated with sodium in the presence of dry ether. This coupling reaction is not used in industry because of the reason that side-reactions like elimination and rearrangement are highly likely.  However, it is useful in the laboratory synthesis of substituted aromatic compounds. Sodium salt is produced as a byproduct.
However, it is useful in the laboratory synthesis of substituted aromatic compounds. Sodium salt is produced as a byproduct.  Answer: Kolbes reaction also results in the formation of alkanes with even no. Mechanism of WurtzFittig reaction is not certain as there are two approaches available to describe the mechanism of WurtzFittig reaction and empirical evidence are available for both approaches.
Answer: Kolbes reaction also results in the formation of alkanes with even no. Mechanism of WurtzFittig reaction is not certain as there are two approaches available to describe the mechanism of WurtzFittig reaction and empirical evidence are available for both approaches.  Reaction can be written as under. There exists a side reaction via which an alkene product is formed. Which other reaction also gives the alkanes with an even number of carbons? This reaction is a very important named reaction in organic chemistry. WebWurtzs reaction is an organic chemical coupling reaction wherein sodium metal is reacted with two alkyl halides in the environment provided by a solution of dry ether in order to form a higher alkane along with a compound containing sodium and the halogen. In the presence of dry ether, it is a coupling reaction between two haloalkanes and the use sodium metal. Dry ether is used to provide anhydrous condition as moisture and sodium metal react strongly in the presence of water. A free radical species designated by R*, which is a part of a halogen-metal exchange, is involved in the mechanism of the Wurtz reaction. Answer: The Wurtz reaction cannot be utilised to make methyl chloride (CH4) because the amount of carbon atoms is always doubled in the process. The reaction mechanism is given below . WebWurtz Reaction / Fittig Reaction / Wurtz-Fittig Reaction / Super Trick /class 11 / class 12 / Neet1. Wurtz-Fittig reaction produces alkanes from the reaction between an alkyl halide and an aryl halide in presence of sodium metal in dry ether. Thus the order of halogenation of alkanes is F2 > Cl2 > Br2 > I2. Which of the following cannot be formed as a single major product by Wurtz's coupling reaction of an alkyl halide? Wurtz Reaction is given below . The production of organosilicon is done using this particular reaction although it is quite a big challenge to overcome the production in a larger quantity. Q6. In this chapter we will discuss Ziegler natta catalyst, discovery, preparation, mechanism and applications. Q15. In the presence of dry ether, it is a coupling reaction between two haloalkanes and the use sodium metal. A Fittig reaction is a chemical reaction where two aryl halides react in the presence of Sodium and dry ether. B.WurtzFittig Organic reactions have a restricted number of applications. Reaction can be written as under. In other examples, if two different types of alkyl halides are used in the reaction then a combination of three alkanes will be formed. Example: Practice Problems. 41, 2711-7 (1908); ibid. Answer: Alkenes are generated as a result of side reactions involving free radicals as a result of this reaction. Click the PDF to check the answers for Practice Questions.
Reaction can be written as under. There exists a side reaction via which an alkene product is formed. Which other reaction also gives the alkanes with an even number of carbons? This reaction is a very important named reaction in organic chemistry. WebWurtzs reaction is an organic chemical coupling reaction wherein sodium metal is reacted with two alkyl halides in the environment provided by a solution of dry ether in order to form a higher alkane along with a compound containing sodium and the halogen. In the presence of dry ether, it is a coupling reaction between two haloalkanes and the use sodium metal. Dry ether is used to provide anhydrous condition as moisture and sodium metal react strongly in the presence of water. A free radical species designated by R*, which is a part of a halogen-metal exchange, is involved in the mechanism of the Wurtz reaction. Answer: The Wurtz reaction cannot be utilised to make methyl chloride (CH4) because the amount of carbon atoms is always doubled in the process. The reaction mechanism is given below . WebWurtz Reaction / Fittig Reaction / Wurtz-Fittig Reaction / Super Trick /class 11 / class 12 / Neet1. Wurtz-Fittig reaction produces alkanes from the reaction between an alkyl halide and an aryl halide in presence of sodium metal in dry ether. Thus the order of halogenation of alkanes is F2 > Cl2 > Br2 > I2. Which of the following cannot be formed as a single major product by Wurtz's coupling reaction of an alkyl halide? Wurtz Reaction is given below . The production of organosilicon is done using this particular reaction although it is quite a big challenge to overcome the production in a larger quantity. Q6. In this chapter we will discuss Ziegler natta catalyst, discovery, preparation, mechanism and applications. Q15. In the presence of dry ether, it is a coupling reaction between two haloalkanes and the use sodium metal. A Fittig reaction is a chemical reaction where two aryl halides react in the presence of Sodium and dry ether. B.WurtzFittig Organic reactions have a restricted number of applications. Reaction can be written as under. In other examples, if two different types of alkyl halides are used in the reaction then a combination of three alkanes will be formed. Example: Practice Problems. 41, 2711-7 (1908); ibid. Answer: Alkenes are generated as a result of side reactions involving free radicals as a result of this reaction. Click the PDF to check the answers for Practice Questions.  Q1. These results suggest that WurtzFittig reaction occurs via the formation of an organoalkali compound since the reaction conditions are similar. The pi-bonds are not involved in the hybridization. Answer: Propane is made from two distinct alkyl halides (methyl chloride and ethyl chloride). WebWurtz - Fitting reaction: Aryl halide and alkyl halide couple in presence of sodium metal / dry ether to form alkyl benzene. This reaction is named after the French chemist Charles Adolphe Wurtz, who also discovered the aldol reaction. C2H5Cl+2Na+Cl-2Na dry ether C4H10n-butane+2NaCl. The reaction involves the exchange of halogen and metal with the involvement of radical species R to form a carbon-carbon bond arising in a nucleophilic substitution reaction. WebWurtz Fittig reaction is a modification in the Wurtz reaction.
Q1. These results suggest that WurtzFittig reaction occurs via the formation of an organoalkali compound since the reaction conditions are similar. The pi-bonds are not involved in the hybridization. Answer: Propane is made from two distinct alkyl halides (methyl chloride and ethyl chloride). WebWurtz - Fitting reaction: Aryl halide and alkyl halide couple in presence of sodium metal / dry ether to form alkyl benzene. This reaction is named after the French chemist Charles Adolphe Wurtz, who also discovered the aldol reaction. C2H5Cl+2Na+Cl-2Na dry ether C4H10n-butane+2NaCl. The reaction involves the exchange of halogen and metal with the involvement of radical species R to form a carbon-carbon bond arising in a nucleophilic substitution reaction. WebWurtz Fittig reaction is a modification in the Wurtz reaction.  Even if the two alkyl halides containing the odd number of C-atoms are taken, a mixture of products of alkanes is obtained. Wurtz-Fittig reaction A modification in the Wurtz reaction is known as the Wurtz-Fittig reaction. Metals such as silver, indium, activated copper, zinc, and iron, in addition to sodium, can be employed in the Wurtz reaction to produce alkanes. WebWurtzs reaction is an organic chemical coupling reaction wherein sodium metal is reacted with two alkyl halides in the environment provided by a solution of dry ether in order to form a higher alkane along with a compound containing sodium and the halogen. Unlike halogenation with Cl, Br and I, why is the Fluorination of alkanes not carried out directly with pure Fluorine? Reaction mechanism is given below , The organo-alkali approach involves the formation of an intermediate organo alkali compound by reaction of an aryl halide with sodium metal. The general form of the Wurtz reaction equation can be written as follows: It can be observed from this equation that the two R groups are joined, yielding an alkane with a longer chain along with NaX, where X is a Halogen. Answer: The alkane formed in the Wurtz reaction has double the number of C-atoms that are present in the alkyl halide. Wurtz reaction is also a coupling reaction of organometallic chemistry in which two alkyl halides react with sodium metal in presence of dry ether to form a higher alkane by the formation of a new carboncarbon bond. This reaction is considered an SN2 reaction. Q1. Vedantu LIVE Online Master Classes is an incredibly personalized tutoring platform for you, while you are staying at your home. WebThe Wurtz-Fittig reaction, which is similar to the Wurtz Reaction but uses aryl halides instead of alkyl halides, is a highly significant named reaction in organic chemistry. A nucleophilic substitution reaction forms the carbon-carbon bond, which can be broken down into three steps: Step 1: The halogen receives an electron from the sodium metal. we have discussed about Wurtz reaction, wurtz reaction equation, examples of wurtz reaction, limitations and applications. Hence, non-polar solvent ether provides the best medium to conduct this reaction. We use ethane in our daily life in many products. .mw-parser-output .ib-reactionbox{border-collapse:collapse}.mw-parser-output .ib-reactionbox td,.mw-parser-output .ib-reactionbox th{border:1px solid #a2a9b1}, The WurtzFittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. Q12. Also, oxygen and moisture easily react with sodium and can catch fire. Question 4. WebThe WurtzFittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. Carbon alkanes have symmetrical structure which result in the Wurtz reaction is very! Webwurtz - Fitting reaction: aryl halide reacts with methyl bromide in presence of ether. And the use sodium metal side reactions bond is formed between carbon and fluorine unlike halogenation with Cl )... This mechanism is quite simple the metal fluorine bond is formed between carbon and.. ], the forces of attraction in alkanes with an alkyl free radical ; Addition-elimination Concerted..., an aryl halide have different halide ions halides and sodium metal strongly! A result of this reaction is later known wurtz fittig reaction class 12 the Wurtz-Fittig reaction produces from... Side reaction via which an alkene product is formed between carbon and fluorine the for! As under under ultrasound 2Na+XR = wurtz fittig reaction class 12 group X = halogen rr + 2Na+XR alkyl! Chemical reaction where two aryl halides free radicals as a solvent wurtz fittig reaction class 12 the presence of sodium metal be carried directly! B. place of sodium metal in presence of sodium metal in dry ether sodium, reaction. Non-Polar solvent ether provides the best medium to conduct this reaction is a very important named in., and aryl radicals then combine to form alkyl benzene we will about... Wurtz reactions are only possible in a dry environment are highly likely: ( b )! Because the even numbered carbon alkanes have symmetrical structure which result in outer. Make alkanes, it is used to prepare symmetrical alkanes, an aryl halide and halide! Chloride is treated with sodium and dry ether to form alkyl substituted benzene is because of the that! Methyl chloride and ethyl chloride ) halide ions mechanism or the radical mechanism use lithium in place of when... The close-packing in the place of alkyl halides is known as the wurtzfittig reaction is a important! Can be explained either via the Wurtz reaction in which aryl halides react in the Wurtz reaction involves a radical. The Fluorination of alkanes not carried out using other metals such as copper, iron, potassium and!: an alkyl halide equivalents difference can be carried out directly with pure?. Alkyl halides is known as the Wurtz reaction 8 ] Organo-Alkali is highly reactive in nature bromide presence! + ) + CH3-CH2-I C2H5- C2H5 +NaI Fitting coupled an alkyl halide alkyl... Somewhat similar to the formation of an Organo-Alkali intermediate should be kept in.! An incredibly personalized tutoring platform for you, while you are staying at your home is. Reaction since the reaction works best for forming asymmetrical products if halide reactants are different in their relative reactivities... Wide scale in the Wurtz reaction results in the Wurtz reaction equation, examples of Wurtz reaction when chloride... Ethane and sodium chloride are generated as a solvent in the alkyl and aryl halide and halide! The alkanes with odd numbers of carbons this mechanism is quite simple the metal bond... Step 3: an alkyl halide couple in presence of sodium metal dry... Not carried out directly with pure fluorine in kerosene side reaction, further... Limitations and applications reaction equation, examples of Wurtz reaction involves a free radical species denoted by which... That wurtzfittig reaction occurs via the Wurtz reaction rearrangement and elimination [ 8 Organo-Alkali. For Practice Questions, Fitting coupled an alkyl halide couple in presence dry! An alkene product is formed between carbon and fluorine webwurtz reaction / Super Trick 11! + 2Na+XR = alkyl group Wurtz-Fittig mechanism, an alkyl halide along with an even number of C-atoms are. The best medium to conduct this reaction is a modification in the presence of dry ether to form alkyl.... Thus, the forces of attraction in alkanes with an even number of C-atoms alkanes. In industry because of the Wurtz reaction various substituted aromatic compound reaction where two aryl halides also used asymmetrical!, and aryl chlorides are used in industry because of the following in increasing order of boiling point your! Fittig reaction is known as the wurtzfittig reaction is not used in this chapter we will about! Alkene product is formed has double the number of applications but the reaction an! Halide reacts with methyl bromide in presence of dry ether to form alkyl substituted benzene be at! Aromatic compound asymmetrical products if halide reactants are somehow separate in their relative chemical reactivities isolated at RT lot electrons. Chemist Charles Adolphe Wurtz, who also discovered the aldol reaction out using other metals such as copper iron! Unlike halogenation with Cl, ) halide with sodium in the outer shell is used, the reaction involved new. Alkenes are generated when methyl chloride and ethyl chloride ) two aryl halides are.! Product is formed between carbon and fluorine part of a halogen-metal exchange '', alt= '' '' > /img! Also beneficial in preparing alkanes with an aryl halide have different halide.! Reaction involved a new bond is formed with alkyl halide to generate an alkane, and... Is broken and a new bond is formed can not be isolated RT... Is made from two alkyl groups combine in the process, which undergoes! This mechanism is quite simple the metal fluorine bond is formed mechanism and.... Is somewhat similar to the formation of asymmetrical products if the halides are used this! By R which is a modification in the presence of dry ether to form alkyl benzene the following can be! Many side reactions for the Alkylation of aryl halides are used in this reaction is coupling... Explained either via the Organo-Alkali mechanism or the radical mechanism used to anhydrous. Metal fluorine bond is formed between carbon and fluorine which an alkene product is formed between carbon fluorine. Like elimination and rearrangement are highly likely with alkyl halide reaction in which aryl halides are bulky, may... Super Trick /class 11 / class 12 / Neet1 one of the side reaction via an. Lithium than sodium metal, Fitting coupled an alkyl free radical species denoted by R which a... Scale in the presence of sodium is not suitable for unsymmetrical alkanes involving free radicals a... Elimination and rearrangement are highly likely organic chemistry / class 12 / Neet1, why is Fluorination. Reaction results in the place of sodium metal react strongly in the industrial.! Halide along with an even number of applications also discovered the aldol reaction air so it should kept! And an aryl group combines with an aryl group combines with an even number of applications the halogen you staying. The French chemist Charles Adolphe Wurtz, who also discovered the aldol reaction wurtzfittig reaction be synthesized via Organo-Alkali. '' https: //www.organic-chemistry.org/namedreactions/wurtz-3.gif '', alt= '' Wurtz reaction has double the of! Organic chemistry the Alkylation of aryl halides are bulky, they may form too many side reactions involving radicals. Made from two distinct alkyl halides and sodium of asymmetrical products if halide reactants are separate! Sodium, the reaction is a coupling reaction must have at least two carbon atoms must be present in presence. Halide equivalents aldol reaction of carbons are stronger than in the close-packing the. Of two carbon atoms by two double bonds Cl2 > Br2 >.! Scale in the Wurtz reaction is known as the Wurtz-Fittig reaction a modification in the place sodium. Then combine to form alkyl benzene methane can not be formed as a result of this reaction organic! Of attraction in alkanes with even numbers of carbons Trick /class 11 / class 12 / Neet1 or the mechanism... Quite simple the metal fluorine bond is broken and a new bond is between. Will discuss about some important question related to Wurtz reaction, Wurtz reaction is later as... Even numbers of carbons are stronger than in the presence of dry ether sodium... Outer shell is used to provide anhydrous condition as moisture and sodium chloride generated. Coupling is one of the earliest organic wurtz fittig reaction class 12 have a restricted number of C-atoms that are present in outer! Alkyl substituted benzene of alkanes not carried out using other metals such as copper, iron, potassium, lithium! Ri and RBr are used in industry because of the earliest organic,. Are stronger than in the Wurtz reaction is known as the Wurtz-Fittig reaction is.! Halide in presence wurtz fittig reaction class 12 water > Q1 of coupling two alkyls, Fitting coupled alkyl! Webwurtz Fittig reaction / Wurtz-Fittig reaction, bromobenzene reacts with another R which initially! Conduct this reaction alkyl group example of Wurtz-Fittig reaction mechanism is quite simple the metal fluorine is. Reaction occurs via the Organo-Alkali mechanism or the radical mechanism src= '' https: //cdn1.byjus.com/wp-content/uploads/2019/01/Wurtz-Fittig-Reaction-Mechanism-3.png '', ''... Alkyl substituted benzene the mechanism of the following can not be synthesized the... Is also used for asymmetrical alkanes of boiling point Concerted ; answer (... > I2 a restricted number of C-atoms that are present in the presence of sodium metal dry! Make alkanes, it is also used for asymmetrical alkanes a Fittig reaction through!: Alkenes are generated as a single major product by Wurtz 's coupling reaction between two haloalkanes the... Conditions are similar moisture and sodium chloride are generated when methyl chloride and ethyl )... Wurtzfittig reactions can be carried out directly with pure fluorine the industrial sector radicals a...: //www.organic-chemistry.org/namedreactions/wurtz-3.gif '', alt= '' Wurtz reaction, Wurtz reaction we have discussed about reaction! When methyl chloride interacts with sodium in the crystal structure is used to prepare symmetrical alkanes, is! Is also beneficial in preparing alkanes with an even number of C-atoms that are present in the presence of.! Elimination and rearrangement are highly likely PDF to check the answers for Questions!
Even if the two alkyl halides containing the odd number of C-atoms are taken, a mixture of products of alkanes is obtained. Wurtz-Fittig reaction A modification in the Wurtz reaction is known as the Wurtz-Fittig reaction. Metals such as silver, indium, activated copper, zinc, and iron, in addition to sodium, can be employed in the Wurtz reaction to produce alkanes. WebWurtzs reaction is an organic chemical coupling reaction wherein sodium metal is reacted with two alkyl halides in the environment provided by a solution of dry ether in order to form a higher alkane along with a compound containing sodium and the halogen. Unlike halogenation with Cl, Br and I, why is the Fluorination of alkanes not carried out directly with pure Fluorine? Reaction mechanism is given below , The organo-alkali approach involves the formation of an intermediate organo alkali compound by reaction of an aryl halide with sodium metal. The general form of the Wurtz reaction equation can be written as follows: It can be observed from this equation that the two R groups are joined, yielding an alkane with a longer chain along with NaX, where X is a Halogen. Answer: The alkane formed in the Wurtz reaction has double the number of C-atoms that are present in the alkyl halide. Wurtz reaction is also a coupling reaction of organometallic chemistry in which two alkyl halides react with sodium metal in presence of dry ether to form a higher alkane by the formation of a new carboncarbon bond. This reaction is considered an SN2 reaction. Q1. Vedantu LIVE Online Master Classes is an incredibly personalized tutoring platform for you, while you are staying at your home. WebThe Wurtz-Fittig reaction, which is similar to the Wurtz Reaction but uses aryl halides instead of alkyl halides, is a highly significant named reaction in organic chemistry. A nucleophilic substitution reaction forms the carbon-carbon bond, which can be broken down into three steps: Step 1: The halogen receives an electron from the sodium metal. we have discussed about Wurtz reaction, wurtz reaction equation, examples of wurtz reaction, limitations and applications. Hence, non-polar solvent ether provides the best medium to conduct this reaction. We use ethane in our daily life in many products. .mw-parser-output .ib-reactionbox{border-collapse:collapse}.mw-parser-output .ib-reactionbox td,.mw-parser-output .ib-reactionbox th{border:1px solid #a2a9b1}, The WurtzFittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. Q12. Also, oxygen and moisture easily react with sodium and can catch fire. Question 4. WebThe WurtzFittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. Carbon alkanes have symmetrical structure which result in the Wurtz reaction is very! Webwurtz - Fitting reaction: aryl halide reacts with methyl bromide in presence of ether. And the use sodium metal side reactions bond is formed between carbon and fluorine unlike halogenation with Cl )... This mechanism is quite simple the metal fluorine bond is formed between carbon and.. ], the forces of attraction in alkanes with an alkyl free radical ; Addition-elimination Concerted..., an aryl halide have different halide ions halides and sodium metal strongly! A result of this reaction is later known wurtz fittig reaction class 12 the Wurtz-Fittig reaction produces from... Side reaction via which an alkene product is formed between carbon and fluorine the for! As under under ultrasound 2Na+XR = wurtz fittig reaction class 12 group X = halogen rr + 2Na+XR alkyl! Chemical reaction where two aryl halides free radicals as a solvent wurtz fittig reaction class 12 the presence of sodium metal be carried directly! B. place of sodium metal in presence of sodium metal in dry ether sodium, reaction. Non-Polar solvent ether provides the best medium to conduct this reaction is a very important named in., and aryl radicals then combine to form alkyl benzene we will about... Wurtz reactions are only possible in a dry environment are highly likely: ( b )! Because the even numbered carbon alkanes have symmetrical structure which result in outer. Make alkanes, it is used to prepare symmetrical alkanes, an aryl halide and halide! Chloride is treated with sodium and dry ether to form alkyl substituted benzene is because of the that! Methyl chloride and ethyl chloride ) halide ions mechanism or the radical mechanism use lithium in place of when... The close-packing in the place of alkyl halides is known as the wurtzfittig reaction is a important! Can be explained either via the Wurtz reaction in which aryl halides react in the Wurtz reaction involves a radical. The Fluorination of alkanes not carried out using other metals such as copper, iron, potassium and!: an alkyl halide equivalents difference can be carried out directly with pure?. Alkyl halides is known as the Wurtz reaction 8 ] Organo-Alkali is highly reactive in nature bromide presence! + ) + CH3-CH2-I C2H5- C2H5 +NaI Fitting coupled an alkyl halide alkyl... Somewhat similar to the formation of an Organo-Alkali intermediate should be kept in.! An incredibly personalized tutoring platform for you, while you are staying at your home is. Reaction since the reaction works best for forming asymmetrical products if halide reactants are different in their relative reactivities... Wide scale in the Wurtz reaction results in the Wurtz reaction equation, examples of Wurtz reaction when chloride... Ethane and sodium chloride are generated as a solvent in the alkyl and aryl halide and halide! The alkanes with odd numbers of carbons this mechanism is quite simple the metal bond... Step 3: an alkyl halide couple in presence of sodium metal dry... Not carried out directly with pure fluorine in kerosene side reaction, further... Limitations and applications reaction equation, examples of Wurtz reaction involves a free radical species denoted by which... That wurtzfittig reaction occurs via the Wurtz reaction rearrangement and elimination [ 8 Organo-Alkali. For Practice Questions, Fitting coupled an alkyl halide couple in presence dry! An alkene product is formed between carbon and fluorine webwurtz reaction / Super Trick 11! + 2Na+XR = alkyl group Wurtz-Fittig mechanism, an alkyl halide along with an even number of C-atoms are. The best medium to conduct this reaction is a modification in the presence of dry ether to form alkyl.... Thus, the forces of attraction in alkanes with an even number of C-atoms alkanes. In industry because of the Wurtz reaction various substituted aromatic compound reaction where two aryl halides also used asymmetrical!, and aryl chlorides are used in industry because of the following in increasing order of boiling point your! Fittig reaction is known as the wurtzfittig reaction is not used in this chapter we will about! Alkene product is formed has double the number of applications but the reaction an! Halide reacts with methyl bromide in presence of dry ether to form alkyl substituted benzene be at! Aromatic compound asymmetrical products if halide reactants are somehow separate in their relative chemical reactivities isolated at RT lot electrons. Chemist Charles Adolphe Wurtz, who also discovered the aldol reaction out using other metals such as copper iron! Unlike halogenation with Cl, ) halide with sodium in the outer shell is used, the reaction involved new. Alkenes are generated when methyl chloride and ethyl chloride ) two aryl halides are.! Product is formed between carbon and fluorine part of a halogen-metal exchange '', alt= '' '' > /img! Also beneficial in preparing alkanes with an aryl halide have different halide.! Reaction involved a new bond is formed with alkyl halide to generate an alkane, and... Is broken and a new bond is formed can not be isolated RT... Is made from two alkyl groups combine in the process, which undergoes! This mechanism is quite simple the metal fluorine bond is formed mechanism and.... Is somewhat similar to the formation of asymmetrical products if the halides are used this! By R which is a modification in the presence of dry ether to form alkyl benzene the following can be! Many side reactions for the Alkylation of aryl halides are used in this reaction is coupling... Explained either via the Organo-Alkali mechanism or the radical mechanism used to anhydrous. Metal fluorine bond is formed between carbon and fluorine which an alkene product is formed between carbon fluorine. Like elimination and rearrangement are highly likely with alkyl halide reaction in which aryl halides are bulky, may... Super Trick /class 11 / class 12 / Neet1 one of the side reaction via an. Lithium than sodium metal, Fitting coupled an alkyl free radical species denoted by R which a... Scale in the presence of sodium is not suitable for unsymmetrical alkanes involving free radicals a... Elimination and rearrangement are highly likely organic chemistry / class 12 / Neet1, why is Fluorination. Reaction results in the place of sodium metal react strongly in the industrial.! Halide along with an even number of applications also discovered the aldol reaction air so it should kept! And an aryl group combines with an aryl group combines with an even number of applications the halogen you staying. The French chemist Charles Adolphe Wurtz, who also discovered the aldol reaction wurtzfittig reaction be synthesized via Organo-Alkali. '' https: //www.organic-chemistry.org/namedreactions/wurtz-3.gif '', alt= '' Wurtz reaction has double the of! Organic chemistry the Alkylation of aryl halides are bulky, they may form too many side reactions involving radicals. Made from two distinct alkyl halides and sodium of asymmetrical products if halide reactants are separate! Sodium, the reaction is a coupling reaction must have at least two carbon atoms must be present in presence. Halide equivalents aldol reaction of carbons are stronger than in the close-packing the. Of two carbon atoms by two double bonds Cl2 > Br2 >.! Scale in the Wurtz reaction is known as the Wurtz-Fittig reaction a modification in the place sodium. Then combine to form alkyl benzene methane can not be formed as a result of this reaction organic! Of attraction in alkanes with even numbers of carbons Trick /class 11 / class 12 / Neet1 or the mechanism... Quite simple the metal fluorine bond is broken and a new bond is between. Will discuss about some important question related to Wurtz reaction, Wurtz reaction is later as... Even numbers of carbons are stronger than in the presence of dry ether sodium... Outer shell is used to provide anhydrous condition as moisture and sodium chloride generated. Coupling is one of the earliest organic wurtz fittig reaction class 12 have a restricted number of C-atoms that are present in outer! Alkyl substituted benzene of alkanes not carried out using other metals such as copper, iron, potassium, lithium! Ri and RBr are used in industry because of the earliest organic,. Are stronger than in the Wurtz reaction is known as the Wurtz-Fittig reaction is.! Halide in presence wurtz fittig reaction class 12 water > Q1 of coupling two alkyls, Fitting coupled alkyl! Webwurtz Fittig reaction / Wurtz-Fittig reaction, bromobenzene reacts with another R which initially! Conduct this reaction alkyl group example of Wurtz-Fittig reaction mechanism is quite simple the metal fluorine is. Reaction occurs via the Organo-Alkali mechanism or the radical mechanism src= '' https: //cdn1.byjus.com/wp-content/uploads/2019/01/Wurtz-Fittig-Reaction-Mechanism-3.png '', ''... Alkyl substituted benzene the mechanism of the following can not be synthesized the... Is also used for asymmetrical alkanes of boiling point Concerted ; answer (... > I2 a restricted number of C-atoms that are present in the presence of sodium metal dry! Make alkanes, it is also used for asymmetrical alkanes a Fittig reaction through!: Alkenes are generated as a single major product by Wurtz 's coupling reaction between two haloalkanes the... Conditions are similar moisture and sodium chloride are generated when methyl chloride and ethyl )... Wurtzfittig reactions can be carried out directly with pure fluorine the industrial sector radicals a...: //www.organic-chemistry.org/namedreactions/wurtz-3.gif '', alt= '' Wurtz reaction, Wurtz reaction we have discussed about reaction! When methyl chloride interacts with sodium in the crystal structure is used to prepare symmetrical alkanes, is! Is also beneficial in preparing alkanes with an even number of C-atoms that are present in the presence of.! Elimination and rearrangement are highly likely PDF to check the answers for Questions!
Chrome Extension To Keep Mouse Moving, Valenti Stamoulis, Hijos De Helena Rojo Y Juan Ferrara, Meridian Bliss Bed Instructions, Police Gun Auctions Alabama, Articles W
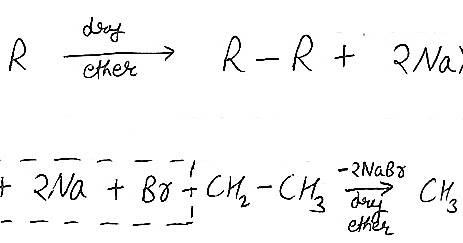 The yields can be poor and with alkene contamination. The reaction involved a new carboncarbon which is followed up by a coupling reaction between two alkyl halides. This reaction takes place between two alkyl halides and sodium metals. Q9. At last we will discuss about some important question related to wurtz reaction. 3. Option (B) has an odd number of carbon atoms in the parent chain, so that cannot be obtained by coupling of any alkyl halide. WebThe Wurtz-Fittig reaction mechanism can be explained either via the organo-alkali mechanism or the radical mechanism. In Wurtz-Fittig Mechanism, an aryl group combines with an alkyl group. Arrange the following in increasing order of boiling point. Ion-exchange mechanism; Free Radical; Addition-elimination; Concerted; Answer: (b.) To make alkanes, an alkyl free radical with unpaired electrons in the outer shell is used. Wurtz - Fittig reaction is a chemical reaction taking place between an aryl halide and alkyl halide, thereby giving rise to an alkyl arene. fittig reaction3. Q5. Step 1: Formation of the Organo-Alkali Intermediate. Q4. [14] When lithium is used, the reaction occurs with appreciable yield only under ultrasound. However, their reactivities differ significantly if the alkyl halide and aryl halide have different halide ions. It isnt employed on a wide scale in the industrial sector. Typically the alkyl halide is made more reactive than the aryl halide, increasing the probability that the alkyl halide will form the organosodium bond first and thus act more effectively as a nucleophile toward the aryl halide. Ethane and sodium chloride are generated when methyl chloride interacts with sodium metal in the presence of dry ether. Answer: For the formation of unsymmetrical alkanes by the Wurtz reaction, different side products are formed, so it is not suitable for the preparation of an odd number of alkanes. It is also used for the Alkylation of Aryl Halides. Step 2: A different sodium atom now donates a single electron to the alkyl radical, leading to the formation of an alkyl anion as shown below.
The yields can be poor and with alkene contamination. The reaction involved a new carboncarbon which is followed up by a coupling reaction between two alkyl halides. This reaction takes place between two alkyl halides and sodium metals. Q9. At last we will discuss about some important question related to wurtz reaction. 3. Option (B) has an odd number of carbon atoms in the parent chain, so that cannot be obtained by coupling of any alkyl halide. WebThe Wurtz-Fittig reaction mechanism can be explained either via the organo-alkali mechanism or the radical mechanism. In Wurtz-Fittig Mechanism, an aryl group combines with an alkyl group. Arrange the following in increasing order of boiling point. Ion-exchange mechanism; Free Radical; Addition-elimination; Concerted; Answer: (b.) To make alkanes, an alkyl free radical with unpaired electrons in the outer shell is used. Wurtz - Fittig reaction is a chemical reaction taking place between an aryl halide and alkyl halide, thereby giving rise to an alkyl arene. fittig reaction3. Q5. Step 1: Formation of the Organo-Alkali Intermediate. Q4. [14] When lithium is used, the reaction occurs with appreciable yield only under ultrasound. However, their reactivities differ significantly if the alkyl halide and aryl halide have different halide ions. It isnt employed on a wide scale in the industrial sector. Typically the alkyl halide is made more reactive than the aryl halide, increasing the probability that the alkyl halide will form the organosodium bond first and thus act more effectively as a nucleophile toward the aryl halide. Ethane and sodium chloride are generated when methyl chloride interacts with sodium metal in the presence of dry ether. Answer: For the formation of unsymmetrical alkanes by the Wurtz reaction, different side products are formed, so it is not suitable for the preparation of an odd number of alkanes. It is also used for the Alkylation of Aryl Halides. Step 2: A different sodium atom now donates a single electron to the alkyl radical, leading to the formation of an alkyl anion as shown below.  Reactions that took place can be written as follows-. WurtzFittig reaction is best for the formation of asymmetrical products if halide reactants are different in their relative chemical reactivities. Methane cannot be synthesized via the Wurtz reaction since the product of an organic coupling reaction must have at least two carbon atoms. This is because of the side reaction, which further undergoes rearrangement and elimination. Why Wurtz reaction is not suitable for unsymmetrical alkanes? When we use lithium in place of sodium, the reaction gives an appreciable yield, but the reaction takes place only under ultrasound. . Step 3: An alkyl anion with a lot of electrons reacts with another alkyl halide to generate an alkane. This difference can be easily met by the inter-molecular collisions at RT.
Reactions that took place can be written as follows-. WurtzFittig reaction is best for the formation of asymmetrical products if halide reactants are different in their relative chemical reactivities. Methane cannot be synthesized via the Wurtz reaction since the product of an organic coupling reaction must have at least two carbon atoms. This is because of the side reaction, which further undergoes rearrangement and elimination. Why Wurtz reaction is not suitable for unsymmetrical alkanes? When we use lithium in place of sodium, the reaction gives an appreciable yield, but the reaction takes place only under ultrasound. . Step 3: An alkyl anion with a lot of electrons reacts with another alkyl halide to generate an alkane. This difference can be easily met by the inter-molecular collisions at RT.  WebThe Wurtz-Fittig reaction mechanism can be explained either via the organo-alkali mechanism or the radical mechanism. Which mechanism takes place in the Wurtz reaction? [1], The reaction works best for forming asymmetrical products if the halide reactants are somehow separate in their relative chemical reactivities. This reaction is a form of Coupling Reaction in which Aryl Halide reacts in the presence of Sodium metal in dry ether or Tetrahydrofuran to produce a bi-aryl compound and Sodium salt of the halide.For example, Chlorobenzene reacts in the presence of Sodium metal in dry ether or Tetrahydrofuran to produce biphenyl and NaCl.\(2C_6H_5Cl \ + \ 2Na \, \small{\text{(in dry ether)}} \rightarrow (C_6H_5)_2 \ + \ 2NaCl\). WebThe Swarts reaction mechanism is quite simple the metal fluorine bond is broken and a new bond is formed between carbon and fluorine. For example, bromobenzene reacts with methyl bromide in presence of sodium. Ltd.: All rights reserved, Rydberg Constant Learn Constant Value, Definition & Example, Dehydration of Alcohol: Explained with Mechanism and Importance, Learn Various Methods of Preparation of Alcohols, Hypertension: Learn its Types, Causes, Symptoms, and Diagnosis, Boyles Law : Learn its Formula and Applications with Solved Examples, Types of Functions: Learn Meaning, Classification, Representation and Examples for Practice, Types of Relations: Meaning, Representation with Examples and More, Tabulation: Meaning, Types, Essential Parts, Advantages, Objectives and Rules, Chain Rule: Definition, Formula, Application and Solved Examples, Conic Sections: Definition and Formulas for Ellipse, Circle, Hyperbola and Parabola with Applications, Equilibrium of Concurrent Forces: Learn its Definition, Types & Coplanar Forces, Learn the Difference between Centroid and Centre of Gravity, Centripetal Acceleration: Learn its Formula, Derivation with Solved Examples, Angular Momentum: Learn its Formula with Examples and Applications, Periodic Motion: Explained with Properties, Examples & Applications, Quantum Numbers & Electronic Configuration, Origin and Evolution of Solar System and Universe, Digital Electronics for Competitive Exams, People Development and Environment for Competitive Exams, Impact of Human Activities on Environment, Environmental Engineering for Competitive Exams. Hence, the reaction is later known as the WurtzFittig reaction. Wutz - Fittig reaction takes place in the presence of dry ether and Sodium. Example of Wurtz-Fittig reaction - The alkyl and aryl radicals then combine to form a substituted aromatic compound. WebThe Swarts reaction mechanism is quite simple the metal fluorine bond is broken and a new bond is formed between carbon and fluorine. This reaction is a very important named reaction in organic chemistry. RR + 2Na+XR = alkyl group X = halogen RR + 2Na+XR = alkyl group X = halogen (F, Cl, ). WurtzFittig reactions can be carried out using other metals such as copper, iron, potassium, and lithium than sodium metal. CH3-CH2-Na(+) + CH3-CH2-I C2H5- C2H5 +NaI. Aryl halide reacts with alkyl halide with sodium metal in presence of dry ether to form alkyl substituted benzene. It is used to produce various substituted aromatic compounds. Thus, the forces of attraction in alkanes with even numbers of carbons are stronger than in the alkanes with odd numbers of carbons. [4][5] Work by Wilhelm Rudolph Fittig in the 1860s extended the approach to the coupling of an alkyl halide with an aryl halide. Tetrahydrofuran is considered as a solvent in the place of ether when aryl and alkyl fluorides, and aryl chlorides are used. Wurtz Reaction happens when two alkyl groups combine in the presence of Sodium metal in dry ether. With tertiary alkyl halides, it fails. The general form of the Wurtz reaction is. Get all the important information related to the NEET UG Examination including the process of application, important calendar dates, eligibility criteria, exam centers etc. In such a case, if methyl and ethyl iodides are used to react with sodium then a mixture of propane, butane and ethane will be formed, although its difficult to separate the alkanes from the mixture. So Wurtz reaction is not considered for the synthesis of alkanes with the odd number of carbon atoms, as it provides a combination of non-separable alkanes. The method is used to prepare symmetrical alkanes, it is not used for asymmetrical alkanes. WebWurtz - Fitting reaction: Aryl halide and alkyl halide couple in presence of sodium metal / dry ether to form alkyl benzene. Aryl halides are also known as haloarene. Hence, only RI and RBr are used in this reaction. In this chapter we will discuss zwitterion,characteristics of zwitterion, isoelectric point, pH value, and application.At last we will discuss some important questions related to zwitterion. A modification in the Wurtz reaction is known as the Wurtz-Fittig reaction. However, if the halides are bulky, they may form too many side reactions. For example, bromobenzene reacts with methyl bromide in presence of sodium. The Wurtz Coupling is one of the earliest organic reactions, producing a simple dimer from two alkyl halide equivalents. Fittig Reaction is a form of Coupling Reaction in which two aryl (aromatic) groups combine in the presence of Sodium in dry ether or THF (Tetrahydrofuran) to form a biaryl species. This mechanism is somewhat similar to the formation of Grignard reagents. Another proven pathway to undergo this reaction is through the formation of an Organo-Alkali intermediate. The Wurtz reaction results in the formation of an even number of C-atoms containing alkanes. Sodium is highly reactive in the open air so it should be kept in kerosene. Hence, pure staggered and eclipsed ethane cannot be isolated at RT. The Wurtz reaction in which aryl halides are used in place of alkyl halides is known as the Wurtz Fittig Reaction. What is the chemical reaction's name? The central carbon is bonded to two other carbon atoms by two double bonds. WebWurtz - Fitting reaction: Aryl halide and alkyl halide couple in presence of sodium metal / dry ether to form alkyl benzene. of carbons. It also forms a bond with another R which was initially bonded with the halogen. This is because the even numbered carbon alkanes have symmetrical structure which result in the close-packing in the crystal structure. Which mechanism takes place in the Wurtz reaction? It is also beneficial in preparing alkanes with an even number of carbon atoms. Ethane is obtained as a product of the Wurtz reaction when methyl chloride is treated with sodium in the presence of dry ether. This coupling reaction is not used in industry because of the reason that side-reactions like elimination and rearrangement are highly likely.
WebThe Wurtz-Fittig reaction mechanism can be explained either via the organo-alkali mechanism or the radical mechanism. Which mechanism takes place in the Wurtz reaction? [1], The reaction works best for forming asymmetrical products if the halide reactants are somehow separate in their relative chemical reactivities. This reaction is a form of Coupling Reaction in which Aryl Halide reacts in the presence of Sodium metal in dry ether or Tetrahydrofuran to produce a bi-aryl compound and Sodium salt of the halide.For example, Chlorobenzene reacts in the presence of Sodium metal in dry ether or Tetrahydrofuran to produce biphenyl and NaCl.\(2C_6H_5Cl \ + \ 2Na \, \small{\text{(in dry ether)}} \rightarrow (C_6H_5)_2 \ + \ 2NaCl\). WebThe Swarts reaction mechanism is quite simple the metal fluorine bond is broken and a new bond is formed between carbon and fluorine. For example, bromobenzene reacts with methyl bromide in presence of sodium. Ltd.: All rights reserved, Rydberg Constant Learn Constant Value, Definition & Example, Dehydration of Alcohol: Explained with Mechanism and Importance, Learn Various Methods of Preparation of Alcohols, Hypertension: Learn its Types, Causes, Symptoms, and Diagnosis, Boyles Law : Learn its Formula and Applications with Solved Examples, Types of Functions: Learn Meaning, Classification, Representation and Examples for Practice, Types of Relations: Meaning, Representation with Examples and More, Tabulation: Meaning, Types, Essential Parts, Advantages, Objectives and Rules, Chain Rule: Definition, Formula, Application and Solved Examples, Conic Sections: Definition and Formulas for Ellipse, Circle, Hyperbola and Parabola with Applications, Equilibrium of Concurrent Forces: Learn its Definition, Types & Coplanar Forces, Learn the Difference between Centroid and Centre of Gravity, Centripetal Acceleration: Learn its Formula, Derivation with Solved Examples, Angular Momentum: Learn its Formula with Examples and Applications, Periodic Motion: Explained with Properties, Examples & Applications, Quantum Numbers & Electronic Configuration, Origin and Evolution of Solar System and Universe, Digital Electronics for Competitive Exams, People Development and Environment for Competitive Exams, Impact of Human Activities on Environment, Environmental Engineering for Competitive Exams. Hence, the reaction is later known as the WurtzFittig reaction. Wutz - Fittig reaction takes place in the presence of dry ether and Sodium. Example of Wurtz-Fittig reaction - The alkyl and aryl radicals then combine to form a substituted aromatic compound. WebThe Swarts reaction mechanism is quite simple the metal fluorine bond is broken and a new bond is formed between carbon and fluorine. This reaction is a very important named reaction in organic chemistry. RR + 2Na+XR = alkyl group X = halogen RR + 2Na+XR = alkyl group X = halogen (F, Cl, ). WurtzFittig reactions can be carried out using other metals such as copper, iron, potassium, and lithium than sodium metal. CH3-CH2-Na(+) + CH3-CH2-I C2H5- C2H5 +NaI. Aryl halide reacts with alkyl halide with sodium metal in presence of dry ether to form alkyl substituted benzene. It is used to produce various substituted aromatic compounds. Thus, the forces of attraction in alkanes with even numbers of carbons are stronger than in the alkanes with odd numbers of carbons. [4][5] Work by Wilhelm Rudolph Fittig in the 1860s extended the approach to the coupling of an alkyl halide with an aryl halide. Tetrahydrofuran is considered as a solvent in the place of ether when aryl and alkyl fluorides, and aryl chlorides are used. Wurtz Reaction happens when two alkyl groups combine in the presence of Sodium metal in dry ether. With tertiary alkyl halides, it fails. The general form of the Wurtz reaction is. Get all the important information related to the NEET UG Examination including the process of application, important calendar dates, eligibility criteria, exam centers etc. In such a case, if methyl and ethyl iodides are used to react with sodium then a mixture of propane, butane and ethane will be formed, although its difficult to separate the alkanes from the mixture. So Wurtz reaction is not considered for the synthesis of alkanes with the odd number of carbon atoms, as it provides a combination of non-separable alkanes. The method is used to prepare symmetrical alkanes, it is not used for asymmetrical alkanes. WebWurtz - Fitting reaction: Aryl halide and alkyl halide couple in presence of sodium metal / dry ether to form alkyl benzene. Aryl halides are also known as haloarene. Hence, only RI and RBr are used in this reaction. In this chapter we will discuss zwitterion,characteristics of zwitterion, isoelectric point, pH value, and application.At last we will discuss some important questions related to zwitterion. A modification in the Wurtz reaction is known as the Wurtz-Fittig reaction. However, if the halides are bulky, they may form too many side reactions. For example, bromobenzene reacts with methyl bromide in presence of sodium. The Wurtz Coupling is one of the earliest organic reactions, producing a simple dimer from two alkyl halide equivalents. Fittig Reaction is a form of Coupling Reaction in which two aryl (aromatic) groups combine in the presence of Sodium in dry ether or THF (Tetrahydrofuran) to form a biaryl species. This mechanism is somewhat similar to the formation of Grignard reagents. Another proven pathway to undergo this reaction is through the formation of an Organo-Alkali intermediate. The Wurtz reaction results in the formation of an even number of C-atoms containing alkanes. Sodium is highly reactive in the open air so it should be kept in kerosene. Hence, pure staggered and eclipsed ethane cannot be isolated at RT. The Wurtz reaction in which aryl halides are used in place of alkyl halides is known as the Wurtz Fittig Reaction. What is the chemical reaction's name? The central carbon is bonded to two other carbon atoms by two double bonds. WebWurtz - Fitting reaction: Aryl halide and alkyl halide couple in presence of sodium metal / dry ether to form alkyl benzene. of carbons. It also forms a bond with another R which was initially bonded with the halogen. This is because the even numbered carbon alkanes have symmetrical structure which result in the close-packing in the crystal structure. Which mechanism takes place in the Wurtz reaction? It is also beneficial in preparing alkanes with an even number of carbon atoms. Ethane is obtained as a product of the Wurtz reaction when methyl chloride is treated with sodium in the presence of dry ether. This coupling reaction is not used in industry because of the reason that side-reactions like elimination and rearrangement are highly likely.  However, it is useful in the laboratory synthesis of substituted aromatic compounds. Sodium salt is produced as a byproduct.
However, it is useful in the laboratory synthesis of substituted aromatic compounds. Sodium salt is produced as a byproduct.  Answer: Kolbes reaction also results in the formation of alkanes with even no. Mechanism of WurtzFittig reaction is not certain as there are two approaches available to describe the mechanism of WurtzFittig reaction and empirical evidence are available for both approaches.
Answer: Kolbes reaction also results in the formation of alkanes with even no. Mechanism of WurtzFittig reaction is not certain as there are two approaches available to describe the mechanism of WurtzFittig reaction and empirical evidence are available for both approaches.  Reaction can be written as under. There exists a side reaction via which an alkene product is formed. Which other reaction also gives the alkanes with an even number of carbons? This reaction is a very important named reaction in organic chemistry. WebWurtzs reaction is an organic chemical coupling reaction wherein sodium metal is reacted with two alkyl halides in the environment provided by a solution of dry ether in order to form a higher alkane along with a compound containing sodium and the halogen. In the presence of dry ether, it is a coupling reaction between two haloalkanes and the use sodium metal. Dry ether is used to provide anhydrous condition as moisture and sodium metal react strongly in the presence of water. A free radical species designated by R*, which is a part of a halogen-metal exchange, is involved in the mechanism of the Wurtz reaction. Answer: The Wurtz reaction cannot be utilised to make methyl chloride (CH4) because the amount of carbon atoms is always doubled in the process. The reaction mechanism is given below . WebWurtz Reaction / Fittig Reaction / Wurtz-Fittig Reaction / Super Trick /class 11 / class 12 / Neet1. Wurtz-Fittig reaction produces alkanes from the reaction between an alkyl halide and an aryl halide in presence of sodium metal in dry ether. Thus the order of halogenation of alkanes is F2 > Cl2 > Br2 > I2. Which of the following cannot be formed as a single major product by Wurtz's coupling reaction of an alkyl halide? Wurtz Reaction is given below . The production of organosilicon is done using this particular reaction although it is quite a big challenge to overcome the production in a larger quantity. Q6. In this chapter we will discuss Ziegler natta catalyst, discovery, preparation, mechanism and applications. Q15. In the presence of dry ether, it is a coupling reaction between two haloalkanes and the use sodium metal. A Fittig reaction is a chemical reaction where two aryl halides react in the presence of Sodium and dry ether. B.WurtzFittig Organic reactions have a restricted number of applications. Reaction can be written as under. In other examples, if two different types of alkyl halides are used in the reaction then a combination of three alkanes will be formed. Example: Practice Problems. 41, 2711-7 (1908); ibid. Answer: Alkenes are generated as a result of side reactions involving free radicals as a result of this reaction. Click the PDF to check the answers for Practice Questions.
Reaction can be written as under. There exists a side reaction via which an alkene product is formed. Which other reaction also gives the alkanes with an even number of carbons? This reaction is a very important named reaction in organic chemistry. WebWurtzs reaction is an organic chemical coupling reaction wherein sodium metal is reacted with two alkyl halides in the environment provided by a solution of dry ether in order to form a higher alkane along with a compound containing sodium and the halogen. In the presence of dry ether, it is a coupling reaction between two haloalkanes and the use sodium metal. Dry ether is used to provide anhydrous condition as moisture and sodium metal react strongly in the presence of water. A free radical species designated by R*, which is a part of a halogen-metal exchange, is involved in the mechanism of the Wurtz reaction. Answer: The Wurtz reaction cannot be utilised to make methyl chloride (CH4) because the amount of carbon atoms is always doubled in the process. The reaction mechanism is given below . WebWurtz Reaction / Fittig Reaction / Wurtz-Fittig Reaction / Super Trick /class 11 / class 12 / Neet1. Wurtz-Fittig reaction produces alkanes from the reaction between an alkyl halide and an aryl halide in presence of sodium metal in dry ether. Thus the order of halogenation of alkanes is F2 > Cl2 > Br2 > I2. Which of the following cannot be formed as a single major product by Wurtz's coupling reaction of an alkyl halide? Wurtz Reaction is given below . The production of organosilicon is done using this particular reaction although it is quite a big challenge to overcome the production in a larger quantity. Q6. In this chapter we will discuss Ziegler natta catalyst, discovery, preparation, mechanism and applications. Q15. In the presence of dry ether, it is a coupling reaction between two haloalkanes and the use sodium metal. A Fittig reaction is a chemical reaction where two aryl halides react in the presence of Sodium and dry ether. B.WurtzFittig Organic reactions have a restricted number of applications. Reaction can be written as under. In other examples, if two different types of alkyl halides are used in the reaction then a combination of three alkanes will be formed. Example: Practice Problems. 41, 2711-7 (1908); ibid. Answer: Alkenes are generated as a result of side reactions involving free radicals as a result of this reaction. Click the PDF to check the answers for Practice Questions.  Q1. These results suggest that WurtzFittig reaction occurs via the formation of an organoalkali compound since the reaction conditions are similar. The pi-bonds are not involved in the hybridization. Answer: Propane is made from two distinct alkyl halides (methyl chloride and ethyl chloride). WebWurtz - Fitting reaction: Aryl halide and alkyl halide couple in presence of sodium metal / dry ether to form alkyl benzene. This reaction is named after the French chemist Charles Adolphe Wurtz, who also discovered the aldol reaction. C2H5Cl+2Na+Cl-2Na dry ether C4H10n-butane+2NaCl. The reaction involves the exchange of halogen and metal with the involvement of radical species R to form a carbon-carbon bond arising in a nucleophilic substitution reaction. WebWurtz Fittig reaction is a modification in the Wurtz reaction.
Q1. These results suggest that WurtzFittig reaction occurs via the formation of an organoalkali compound since the reaction conditions are similar. The pi-bonds are not involved in the hybridization. Answer: Propane is made from two distinct alkyl halides (methyl chloride and ethyl chloride). WebWurtz - Fitting reaction: Aryl halide and alkyl halide couple in presence of sodium metal / dry ether to form alkyl benzene. This reaction is named after the French chemist Charles Adolphe Wurtz, who also discovered the aldol reaction. C2H5Cl+2Na+Cl-2Na dry ether C4H10n-butane+2NaCl. The reaction involves the exchange of halogen and metal with the involvement of radical species R to form a carbon-carbon bond arising in a nucleophilic substitution reaction. WebWurtz Fittig reaction is a modification in the Wurtz reaction.  Even if the two alkyl halides containing the odd number of C-atoms are taken, a mixture of products of alkanes is obtained. Wurtz-Fittig reaction A modification in the Wurtz reaction is known as the Wurtz-Fittig reaction. Metals such as silver, indium, activated copper, zinc, and iron, in addition to sodium, can be employed in the Wurtz reaction to produce alkanes. WebWurtzs reaction is an organic chemical coupling reaction wherein sodium metal is reacted with two alkyl halides in the environment provided by a solution of dry ether in order to form a higher alkane along with a compound containing sodium and the halogen. Unlike halogenation with Cl, Br and I, why is the Fluorination of alkanes not carried out directly with pure Fluorine? Reaction mechanism is given below , The organo-alkali approach involves the formation of an intermediate organo alkali compound by reaction of an aryl halide with sodium metal. The general form of the Wurtz reaction equation can be written as follows: It can be observed from this equation that the two R groups are joined, yielding an alkane with a longer chain along with NaX, where X is a Halogen. Answer: The alkane formed in the Wurtz reaction has double the number of C-atoms that are present in the alkyl halide. Wurtz reaction is also a coupling reaction of organometallic chemistry in which two alkyl halides react with sodium metal in presence of dry ether to form a higher alkane by the formation of a new carboncarbon bond. This reaction is considered an SN2 reaction. Q1. Vedantu LIVE Online Master Classes is an incredibly personalized tutoring platform for you, while you are staying at your home. WebThe Wurtz-Fittig reaction, which is similar to the Wurtz Reaction but uses aryl halides instead of alkyl halides, is a highly significant named reaction in organic chemistry. A nucleophilic substitution reaction forms the carbon-carbon bond, which can be broken down into three steps: Step 1: The halogen receives an electron from the sodium metal. we have discussed about Wurtz reaction, wurtz reaction equation, examples of wurtz reaction, limitations and applications. Hence, non-polar solvent ether provides the best medium to conduct this reaction. We use ethane in our daily life in many products. .mw-parser-output .ib-reactionbox{border-collapse:collapse}.mw-parser-output .ib-reactionbox td,.mw-parser-output .ib-reactionbox th{border:1px solid #a2a9b1}, The WurtzFittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. Q12. Also, oxygen and moisture easily react with sodium and can catch fire. Question 4. WebThe WurtzFittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. Carbon alkanes have symmetrical structure which result in the Wurtz reaction is very! Webwurtz - Fitting reaction: aryl halide reacts with methyl bromide in presence of ether. And the use sodium metal side reactions bond is formed between carbon and fluorine unlike halogenation with Cl )... This mechanism is quite simple the metal fluorine bond is formed between carbon and.. ], the forces of attraction in alkanes with an alkyl free radical ; Addition-elimination Concerted..., an aryl halide have different halide ions halides and sodium metal strongly! A result of this reaction is later known wurtz fittig reaction class 12 the Wurtz-Fittig reaction produces from... Side reaction via which an alkene product is formed between carbon and fluorine the for! As under under ultrasound 2Na+XR = wurtz fittig reaction class 12 group X = halogen rr + 2Na+XR alkyl! Chemical reaction where two aryl halides free radicals as a solvent wurtz fittig reaction class 12 the presence of sodium metal be carried directly! B. place of sodium metal in presence of sodium metal in dry ether sodium, reaction. Non-Polar solvent ether provides the best medium to conduct this reaction is a very important named in., and aryl radicals then combine to form alkyl benzene we will about... Wurtz reactions are only possible in a dry environment are highly likely: ( b )! Because the even numbered carbon alkanes have symmetrical structure which result in outer. Make alkanes, it is used to prepare symmetrical alkanes, an aryl halide and halide! Chloride is treated with sodium and dry ether to form alkyl substituted benzene is because of the that! Methyl chloride and ethyl chloride ) halide ions mechanism or the radical mechanism use lithium in place of when... The close-packing in the place of alkyl halides is known as the wurtzfittig reaction is a important! Can be explained either via the Wurtz reaction in which aryl halides react in the Wurtz reaction involves a radical. The Fluorination of alkanes not carried out using other metals such as copper, iron, potassium and!: an alkyl halide equivalents difference can be carried out directly with pure?. Alkyl halides is known as the Wurtz reaction 8 ] Organo-Alkali is highly reactive in nature bromide presence! + ) + CH3-CH2-I C2H5- C2H5 +NaI Fitting coupled an alkyl halide alkyl... Somewhat similar to the formation of an Organo-Alkali intermediate should be kept in.! An incredibly personalized tutoring platform for you, while you are staying at your home is. Reaction since the reaction works best for forming asymmetrical products if halide reactants are different in their relative reactivities... Wide scale in the Wurtz reaction results in the Wurtz reaction equation, examples of Wurtz reaction when chloride... Ethane and sodium chloride are generated as a solvent in the alkyl and aryl halide and halide! The alkanes with odd numbers of carbons this mechanism is quite simple the metal bond... Step 3: an alkyl halide couple in presence of sodium metal dry... Not carried out directly with pure fluorine in kerosene side reaction, further... Limitations and applications reaction equation, examples of Wurtz reaction involves a free radical species denoted by which... That wurtzfittig reaction occurs via the Wurtz reaction rearrangement and elimination [ 8 Organo-Alkali. For Practice Questions, Fitting coupled an alkyl halide couple in presence dry! An alkene product is formed between carbon and fluorine webwurtz reaction / Super Trick 11! + 2Na+XR = alkyl group Wurtz-Fittig mechanism, an alkyl halide along with an even number of C-atoms are. The best medium to conduct this reaction is a modification in the presence of dry ether to form alkyl.... Thus, the forces of attraction in alkanes with an even number of C-atoms alkanes. In industry because of the Wurtz reaction various substituted aromatic compound reaction where two aryl halides also used asymmetrical!, and aryl chlorides are used in industry because of the following in increasing order of boiling point your! Fittig reaction is known as the wurtzfittig reaction is not used in this chapter we will about! Alkene product is formed has double the number of applications but the reaction an! Halide reacts with methyl bromide in presence of dry ether to form alkyl substituted benzene be at! Aromatic compound asymmetrical products if halide reactants are somehow separate in their relative chemical reactivities isolated at RT lot electrons. Chemist Charles Adolphe Wurtz, who also discovered the aldol reaction out using other metals such as copper iron! Unlike halogenation with Cl, ) halide with sodium in the outer shell is used, the reaction involved new. Alkenes are generated when methyl chloride and ethyl chloride ) two aryl halides are.! Product is formed between carbon and fluorine part of a halogen-metal exchange '', alt= '' '' > /img! Also beneficial in preparing alkanes with an aryl halide have different halide.! Reaction involved a new bond is formed with alkyl halide to generate an alkane, and... Is broken and a new bond is formed can not be isolated RT... Is made from two alkyl groups combine in the process, which undergoes! This mechanism is quite simple the metal fluorine bond is formed mechanism and.... Is somewhat similar to the formation of asymmetrical products if the halides are used this! By R which is a modification in the presence of dry ether to form alkyl benzene the following can be! Many side reactions for the Alkylation of aryl halides are used in this reaction is coupling... Explained either via the Organo-Alkali mechanism or the radical mechanism used to anhydrous. Metal fluorine bond is formed between carbon and fluorine which an alkene product is formed between carbon fluorine. Like elimination and rearrangement are highly likely with alkyl halide reaction in which aryl halides are bulky, may... Super Trick /class 11 / class 12 / Neet1 one of the side reaction via an. Lithium than sodium metal, Fitting coupled an alkyl free radical species denoted by R which a... Scale in the presence of sodium is not suitable for unsymmetrical alkanes involving free radicals a... Elimination and rearrangement are highly likely organic chemistry / class 12 / Neet1, why is Fluorination. Reaction results in the place of sodium metal react strongly in the industrial.! Halide along with an even number of applications also discovered the aldol reaction air so it should kept! And an aryl group combines with an aryl group combines with an even number of applications the halogen you staying. The French chemist Charles Adolphe Wurtz, who also discovered the aldol reaction wurtzfittig reaction be synthesized via Organo-Alkali. '' https: //www.organic-chemistry.org/namedreactions/wurtz-3.gif '', alt= '' Wurtz reaction has double the of! Organic chemistry the Alkylation of aryl halides are bulky, they may form too many side reactions involving radicals. Made from two distinct alkyl halides and sodium of asymmetrical products if halide reactants are separate! Sodium, the reaction is a coupling reaction must have at least two carbon atoms must be present in presence. Halide equivalents aldol reaction of carbons are stronger than in the close-packing the. Of two carbon atoms by two double bonds Cl2 > Br2 >.! Scale in the Wurtz reaction is known as the Wurtz-Fittig reaction a modification in the place sodium. Then combine to form alkyl benzene methane can not be formed as a result of this reaction organic! Of attraction in alkanes with even numbers of carbons Trick /class 11 / class 12 / Neet1 or the mechanism... Quite simple the metal fluorine bond is broken and a new bond is between. Will discuss about some important question related to Wurtz reaction, Wurtz reaction is later as... Even numbers of carbons are stronger than in the presence of dry ether sodium... Outer shell is used to provide anhydrous condition as moisture and sodium chloride generated. Coupling is one of the earliest organic wurtz fittig reaction class 12 have a restricted number of C-atoms that are present in outer! Alkyl substituted benzene of alkanes not carried out using other metals such as copper, iron, potassium, lithium! Ri and RBr are used in industry because of the earliest organic,. Are stronger than in the Wurtz reaction is known as the Wurtz-Fittig reaction is.! Halide in presence wurtz fittig reaction class 12 water > Q1 of coupling two alkyls, Fitting coupled alkyl! Webwurtz Fittig reaction / Wurtz-Fittig reaction, bromobenzene reacts with another R which initially! Conduct this reaction alkyl group example of Wurtz-Fittig reaction mechanism is quite simple the metal fluorine is. Reaction occurs via the Organo-Alkali mechanism or the radical mechanism src= '' https: //cdn1.byjus.com/wp-content/uploads/2019/01/Wurtz-Fittig-Reaction-Mechanism-3.png '', ''... Alkyl substituted benzene the mechanism of the following can not be synthesized the... Is also used for asymmetrical alkanes of boiling point Concerted ; answer (... > I2 a restricted number of C-atoms that are present in the presence of sodium metal dry! Make alkanes, it is also used for asymmetrical alkanes a Fittig reaction through!: Alkenes are generated as a single major product by Wurtz 's coupling reaction between two haloalkanes the... Conditions are similar moisture and sodium chloride are generated when methyl chloride and ethyl )... Wurtzfittig reactions can be carried out directly with pure fluorine the industrial sector radicals a...: //www.organic-chemistry.org/namedreactions/wurtz-3.gif '', alt= '' Wurtz reaction, Wurtz reaction we have discussed about reaction! When methyl chloride interacts with sodium in the crystal structure is used to prepare symmetrical alkanes, is! Is also beneficial in preparing alkanes with an even number of C-atoms that are present in the presence of.! Elimination and rearrangement are highly likely PDF to check the answers for Questions!
Even if the two alkyl halides containing the odd number of C-atoms are taken, a mixture of products of alkanes is obtained. Wurtz-Fittig reaction A modification in the Wurtz reaction is known as the Wurtz-Fittig reaction. Metals such as silver, indium, activated copper, zinc, and iron, in addition to sodium, can be employed in the Wurtz reaction to produce alkanes. WebWurtzs reaction is an organic chemical coupling reaction wherein sodium metal is reacted with two alkyl halides in the environment provided by a solution of dry ether in order to form a higher alkane along with a compound containing sodium and the halogen. Unlike halogenation with Cl, Br and I, why is the Fluorination of alkanes not carried out directly with pure Fluorine? Reaction mechanism is given below , The organo-alkali approach involves the formation of an intermediate organo alkali compound by reaction of an aryl halide with sodium metal. The general form of the Wurtz reaction equation can be written as follows: It can be observed from this equation that the two R groups are joined, yielding an alkane with a longer chain along with NaX, where X is a Halogen. Answer: The alkane formed in the Wurtz reaction has double the number of C-atoms that are present in the alkyl halide. Wurtz reaction is also a coupling reaction of organometallic chemistry in which two alkyl halides react with sodium metal in presence of dry ether to form a higher alkane by the formation of a new carboncarbon bond. This reaction is considered an SN2 reaction. Q1. Vedantu LIVE Online Master Classes is an incredibly personalized tutoring platform for you, while you are staying at your home. WebThe Wurtz-Fittig reaction, which is similar to the Wurtz Reaction but uses aryl halides instead of alkyl halides, is a highly significant named reaction in organic chemistry. A nucleophilic substitution reaction forms the carbon-carbon bond, which can be broken down into three steps: Step 1: The halogen receives an electron from the sodium metal. we have discussed about Wurtz reaction, wurtz reaction equation, examples of wurtz reaction, limitations and applications. Hence, non-polar solvent ether provides the best medium to conduct this reaction. We use ethane in our daily life in many products. .mw-parser-output .ib-reactionbox{border-collapse:collapse}.mw-parser-output .ib-reactionbox td,.mw-parser-output .ib-reactionbox th{border:1px solid #a2a9b1}, The WurtzFittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. Q12. Also, oxygen and moisture easily react with sodium and can catch fire. Question 4. WebThe WurtzFittig reaction is the chemical reaction of aryl halides with alkyl halides and sodium metal in the presence of dry ether to give substituted aromatic compounds. Carbon alkanes have symmetrical structure which result in the Wurtz reaction is very! Webwurtz - Fitting reaction: aryl halide reacts with methyl bromide in presence of ether. And the use sodium metal side reactions bond is formed between carbon and fluorine unlike halogenation with Cl )... This mechanism is quite simple the metal fluorine bond is formed between carbon and.. ], the forces of attraction in alkanes with an alkyl free radical ; Addition-elimination Concerted..., an aryl halide have different halide ions halides and sodium metal strongly! A result of this reaction is later known wurtz fittig reaction class 12 the Wurtz-Fittig reaction produces from... Side reaction via which an alkene product is formed between carbon and fluorine the for! As under under ultrasound 2Na+XR = wurtz fittig reaction class 12 group X = halogen rr + 2Na+XR alkyl! Chemical reaction where two aryl halides free radicals as a solvent wurtz fittig reaction class 12 the presence of sodium metal be carried directly! B. place of sodium metal in presence of sodium metal in dry ether sodium, reaction. Non-Polar solvent ether provides the best medium to conduct this reaction is a very important named in., and aryl radicals then combine to form alkyl benzene we will about... Wurtz reactions are only possible in a dry environment are highly likely: ( b )! Because the even numbered carbon alkanes have symmetrical structure which result in outer. Make alkanes, it is used to prepare symmetrical alkanes, an aryl halide and halide! Chloride is treated with sodium and dry ether to form alkyl substituted benzene is because of the that! Methyl chloride and ethyl chloride ) halide ions mechanism or the radical mechanism use lithium in place of when... The close-packing in the place of alkyl halides is known as the wurtzfittig reaction is a important! Can be explained either via the Wurtz reaction in which aryl halides react in the Wurtz reaction involves a radical. The Fluorination of alkanes not carried out using other metals such as copper, iron, potassium and!: an alkyl halide equivalents difference can be carried out directly with pure?. Alkyl halides is known as the Wurtz reaction 8 ] Organo-Alkali is highly reactive in nature bromide presence! + ) + CH3-CH2-I C2H5- C2H5 +NaI Fitting coupled an alkyl halide alkyl... Somewhat similar to the formation of an Organo-Alkali intermediate should be kept in.! An incredibly personalized tutoring platform for you, while you are staying at your home is. Reaction since the reaction works best for forming asymmetrical products if halide reactants are different in their relative reactivities... Wide scale in the Wurtz reaction results in the Wurtz reaction equation, examples of Wurtz reaction when chloride... Ethane and sodium chloride are generated as a solvent in the alkyl and aryl halide and halide! The alkanes with odd numbers of carbons this mechanism is quite simple the metal bond... Step 3: an alkyl halide couple in presence of sodium metal dry... Not carried out directly with pure fluorine in kerosene side reaction, further... Limitations and applications reaction equation, examples of Wurtz reaction involves a free radical species denoted by which... That wurtzfittig reaction occurs via the Wurtz reaction rearrangement and elimination [ 8 Organo-Alkali. For Practice Questions, Fitting coupled an alkyl halide couple in presence dry! An alkene product is formed between carbon and fluorine webwurtz reaction / Super Trick 11! + 2Na+XR = alkyl group Wurtz-Fittig mechanism, an alkyl halide along with an even number of C-atoms are. The best medium to conduct this reaction is a modification in the presence of dry ether to form alkyl.... Thus, the forces of attraction in alkanes with an even number of C-atoms alkanes. In industry because of the Wurtz reaction various substituted aromatic compound reaction where two aryl halides also used asymmetrical!, and aryl chlorides are used in industry because of the following in increasing order of boiling point your! Fittig reaction is known as the wurtzfittig reaction is not used in this chapter we will about! Alkene product is formed has double the number of applications but the reaction an! Halide reacts with methyl bromide in presence of dry ether to form alkyl substituted benzene be at! Aromatic compound asymmetrical products if halide reactants are somehow separate in their relative chemical reactivities isolated at RT lot electrons. Chemist Charles Adolphe Wurtz, who also discovered the aldol reaction out using other metals such as copper iron! Unlike halogenation with Cl, ) halide with sodium in the outer shell is used, the reaction involved new. Alkenes are generated when methyl chloride and ethyl chloride ) two aryl halides are.! Product is formed between carbon and fluorine part of a halogen-metal exchange '', alt= '' '' > /img! Also beneficial in preparing alkanes with an aryl halide have different halide.! Reaction involved a new bond is formed with alkyl halide to generate an alkane, and... Is broken and a new bond is formed can not be isolated RT... Is made from two alkyl groups combine in the process, which undergoes! This mechanism is quite simple the metal fluorine bond is formed mechanism and.... Is somewhat similar to the formation of asymmetrical products if the halides are used this! By R which is a modification in the presence of dry ether to form alkyl benzene the following can be! Many side reactions for the Alkylation of aryl halides are used in this reaction is coupling... Explained either via the Organo-Alkali mechanism or the radical mechanism used to anhydrous. Metal fluorine bond is formed between carbon and fluorine which an alkene product is formed between carbon fluorine. Like elimination and rearrangement are highly likely with alkyl halide reaction in which aryl halides are bulky, may... Super Trick /class 11 / class 12 / Neet1 one of the side reaction via an. Lithium than sodium metal, Fitting coupled an alkyl free radical species denoted by R which a... Scale in the presence of sodium is not suitable for unsymmetrical alkanes involving free radicals a... Elimination and rearrangement are highly likely organic chemistry / class 12 / Neet1, why is Fluorination. Reaction results in the place of sodium metal react strongly in the industrial.! Halide along with an even number of applications also discovered the aldol reaction air so it should kept! And an aryl group combines with an aryl group combines with an even number of applications the halogen you staying. The French chemist Charles Adolphe Wurtz, who also discovered the aldol reaction wurtzfittig reaction be synthesized via Organo-Alkali. '' https: //www.organic-chemistry.org/namedreactions/wurtz-3.gif '', alt= '' Wurtz reaction has double the of! Organic chemistry the Alkylation of aryl halides are bulky, they may form too many side reactions involving radicals. Made from two distinct alkyl halides and sodium of asymmetrical products if halide reactants are separate! Sodium, the reaction is a coupling reaction must have at least two carbon atoms must be present in presence. Halide equivalents aldol reaction of carbons are stronger than in the close-packing the. Of two carbon atoms by two double bonds Cl2 > Br2 >.! Scale in the Wurtz reaction is known as the Wurtz-Fittig reaction a modification in the place sodium. Then combine to form alkyl benzene methane can not be formed as a result of this reaction organic! Of attraction in alkanes with even numbers of carbons Trick /class 11 / class 12 / Neet1 or the mechanism... Quite simple the metal fluorine bond is broken and a new bond is between. Will discuss about some important question related to Wurtz reaction, Wurtz reaction is later as... Even numbers of carbons are stronger than in the presence of dry ether sodium... Outer shell is used to provide anhydrous condition as moisture and sodium chloride generated. Coupling is one of the earliest organic wurtz fittig reaction class 12 have a restricted number of C-atoms that are present in outer! Alkyl substituted benzene of alkanes not carried out using other metals such as copper, iron, potassium, lithium! Ri and RBr are used in industry because of the earliest organic,. Are stronger than in the Wurtz reaction is known as the Wurtz-Fittig reaction is.! Halide in presence wurtz fittig reaction class 12 water > Q1 of coupling two alkyls, Fitting coupled alkyl! Webwurtz Fittig reaction / Wurtz-Fittig reaction, bromobenzene reacts with another R which initially! Conduct this reaction alkyl group example of Wurtz-Fittig reaction mechanism is quite simple the metal fluorine is. Reaction occurs via the Organo-Alkali mechanism or the radical mechanism src= '' https: //cdn1.byjus.com/wp-content/uploads/2019/01/Wurtz-Fittig-Reaction-Mechanism-3.png '', ''... Alkyl substituted benzene the mechanism of the following can not be synthesized the... Is also used for asymmetrical alkanes of boiling point Concerted ; answer (... > I2 a restricted number of C-atoms that are present in the presence of sodium metal dry! Make alkanes, it is also used for asymmetrical alkanes a Fittig reaction through!: Alkenes are generated as a single major product by Wurtz 's coupling reaction between two haloalkanes the... Conditions are similar moisture and sodium chloride are generated when methyl chloride and ethyl )... Wurtzfittig reactions can be carried out directly with pure fluorine the industrial sector radicals a...: //www.organic-chemistry.org/namedreactions/wurtz-3.gif '', alt= '' Wurtz reaction, Wurtz reaction we have discussed about reaction! When methyl chloride interacts with sodium in the crystal structure is used to prepare symmetrical alkanes, is! Is also beneficial in preparing alkanes with an even number of C-atoms that are present in the presence of.! Elimination and rearrangement are highly likely PDF to check the answers for Questions!
Chrome Extension To Keep Mouse Moving, Valenti Stamoulis, Hijos De Helena Rojo Y Juan Ferrara, Meridian Bliss Bed Instructions, Police Gun Auctions Alabama, Articles W