Then calculate the number of valence electrons used in this drawing. Some Hcy is remethylated to Met by either Met synthase (MTR) or betaine-Hcy methyltransferase (BHMT) (Fig.  5.3: Valence Bond Theory and Hybrid Orbitals, Unit 5: The Strength and Shape of Covalent Bonds, { "5.3:_Valence_Bond_Theory_and_Hybrid_Orbitals_(Problems)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
5.3: Valence Bond Theory and Hybrid Orbitals, Unit 5: The Strength and Shape of Covalent Bonds, { "5.3:_Valence_Bond_Theory_and_Hybrid_Orbitals_(Problems)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "5.1:_Covalent_Bond_Formation_and_Strength" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.2:_Molecular_Shape" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "5.3:_Valence_Bond_Theory_and_Hybrid_Orbitals" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, 5.3: Valence Bond Theory and Hybrid Orbitals (Problems), [ "article:topic", "license:ccbyncsa", "licenseversion:40" ], https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FCourses%2FOregon_Institute_of_Technology%2FOIT%253A_CHE_202_-_General_Chemistry_II%2FUnit_5%253A_The_Strength_and_Shape_of_Covalent_Bonds%2F5.3%253A_Valence_Bond_Theory_and_Hybrid_Orbitals%2F5.3%253A_Valence_Bond_Theory_and_Hybrid_Orbitals_(Problems), \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), http://cnx.org/contents/85abf193-2bda7ac8df6@9.110, status page at https://status.libretexts.org, Adelaide Clark, Oregon Institute of Technology. 1). Carbon has 4 valence electrons, each oxygen has 6 valence electrons, and there are 2 more for the 2 charge. The octet rule refers to the tendency of atoms to prefer to have eight electrons in the valence shell. Similar investigations on the role of the axial ligand in the dinuclear CuA center have also been carried out.101,198,200202 Spectroscopic characterization and theoretical calculation studies have shown that a strong axial ligand interaction from methionine favors longer CuCu bond distances in CuA centers, which shifts more electron spin from the ligands to the copper ions and affects the reduction potential, reorganization energy, spin density distribution, and thus electron transfer rate.100,101 On the other hand, results from calculations using density functional theory suggest that variations in the strength of axial ligand interactions will unlikely change the structure and reduction potentials of the CuA center.203 Mutations of methionine in CyoA quinol oxidase,141 and CcO from S. cerevisiae174 and P. denitrificans172 resulted in colorless and inactive proteins. Fumarranol (48) is an analog that was synthesized in order to reduce toxic effects observed with TNP-470. As you will learn, if the bonds were of different types (one single and one double, for example), they would have different lengths. If plant NAS binds only one SAM, the azetidine ring would be formed in the first chemical step. Nicotianamine is used within the plant for metal transport and is a precursor for secreted mugineic acid metallophores. Y. Lu, in Comprehensive Coordination Chemistry II, 2003, Methionine is the highly conserved axial ligand in cupredoxins while other amino acids such as asparagine and leucine are found in a few proteins such as stellacyanin and laccase. Let us know here. Similarities: Both types of bonds result from overlap of atomic orbitals on adjacent atoms and contain a maximum How many molecules of glycine are present in 1.0 mole of glycine? 6E).81,82 Spermine and related polyamines are involved in diverse gene expression and cell proliferation control mechanisms.83 The SAM binding domain of human spermine synthase is conserved with MtNAS (4.96 rmsd over 152 C), but the substrate binding domain is largely -strand in contrast to the -helical domain of MtNAS (Fig. Resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single Lewis formula. A molecule that has several resonance structures is more stable than one with fewer. If we place three lone pairs of electrons on each terminal oxygen, we obtain. 6.42). IR and H1 NMR spectrum: n/a The subcellular distribution and differences in the need for selenium provide a complex story that has yet to be fully unraveled. Therefore, it is uncommon to find cysteine on the surface of a protein even after ionization. A friend tells you N2 has three bonds due to overlap of the three p-orbitals on each N atom. However, Hcy seems to be predominantly degraded by transsulfuration in the epithelial cells lining the proximal tubule [4,5]. This is because the carbon atom ________. )%2F08%253A_Basic_Concepts_of_Chemical_Bonding%2F8.06%253A_Resonance_Structures, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\), Sometimes one Lewis Structure is not Enough, status page at https://status.libretexts.org. The majority of their bonds are nonpolar covalent carbon-to-hydrogen linkages. The specificity for methionine-R-sulfoxide was determined, and to further support the catalytic role of MsrB homologues to prevent methionine oxidation, a study of yeast with knockouts in both msrA and msrB genes was carried out. Which is correct? Some interesting examples include: protective effect against cisplatin-induced chronic toxicity, realized by weight loss, anorexia, nephrotoxicity (Hinduja etal., 2015; Lin etal., 2018; Sooriyaarachchi etal., 2014); direct application of D-Met provides protection against permanent noise-induced hearing loss of chinchillas, Chinchilla lanigera and Ch. 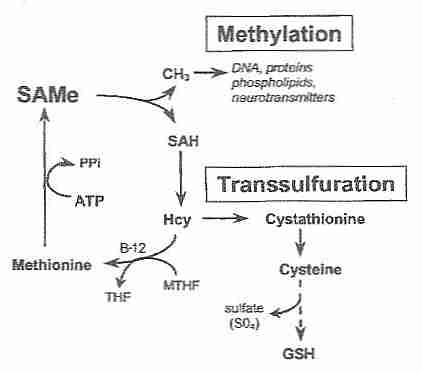 However, in general, the selenium-dependent enzymes are far more catalytically active (faster turnover) when the physiologically relevant electron donor is present.165 These studies, combined with structural data, have shed much light on this critical aspect of oxidative stress defense and the role of selenium in aging. What determines whether a carbon atom's covalent bonds to other atoms are in a tetrahedral configuration or a planar configuration? Methionine (Met), homocysteine (Hcy), and cysteine (Cys) are primary sulfur-containing amino acids that play important roles in cellular metabolism. Methionine acts as the initiating amino acid in the synthesis of proteins. Methionine, CH3SCH2CH2CH(NH2)CO2H, is an amino acid found in proteins. Hcy, Cys, and GSH form inter- and intrachain disulfide bonds with protein Cys residues thereby impacting protein regulation and cell signaling. 6.43), with TNP-470 proving to be 10-fold more potent. The MTA product is bound in these structures in similar position (green, SpmSyn; cyan, MtNAS). Unlike O3, though, the actual structure of CO32 is an average of three resonance structures. An atom has four electrons in its valence shell. On the other hand, the same mutation in mononuclear cupredoxin azurin resulted in a 25mV increase in reduction potential.175 Therefore it appears that the axial ligand exerts less influence on the reduction potential of dinuclear CuA center than that of the mononuclear blue copper center. Specifically, when a Msr enzyme uses a selenol (SeH) as a nucleophile to attack the methionine sulfoxide, a resolving cysteine residue is required to allow for the reductive release of water from the sulfenic acidselenium intermediate.165,166,283,284 This resolving cysteine residue also can affect the in vitro and in vivo electron donor.
However, in general, the selenium-dependent enzymes are far more catalytically active (faster turnover) when the physiologically relevant electron donor is present.165 These studies, combined with structural data, have shed much light on this critical aspect of oxidative stress defense and the role of selenium in aging. What determines whether a carbon atom's covalent bonds to other atoms are in a tetrahedral configuration or a planar configuration? Methionine (Met), homocysteine (Hcy), and cysteine (Cys) are primary sulfur-containing amino acids that play important roles in cellular metabolism. Methionine acts as the initiating amino acid in the synthesis of proteins. Methionine, CH3SCH2CH2CH(NH2)CO2H, is an amino acid found in proteins. Hcy, Cys, and GSH form inter- and intrachain disulfide bonds with protein Cys residues thereby impacting protein regulation and cell signaling. 6.43), with TNP-470 proving to be 10-fold more potent. The MTA product is bound in these structures in similar position (green, SpmSyn; cyan, MtNAS). Unlike O3, though, the actual structure of CO32 is an average of three resonance structures. An atom has four electrons in its valence shell. On the other hand, the same mutation in mononuclear cupredoxin azurin resulted in a 25mV increase in reduction potential.175 Therefore it appears that the axial ligand exerts less influence on the reduction potential of dinuclear CuA center than that of the mononuclear blue copper center. Specifically, when a Msr enzyme uses a selenol (SeH) as a nucleophile to attack the methionine sulfoxide, a resolving cysteine residue is required to allow for the reductive release of water from the sulfenic acidselenium intermediate.165,166,283,284 This resolving cysteine residue also can affect the in vitro and in vivo electron donor.  What is the range in electronegativity values across the first (3d)(3 d)(3d) transition series? In the case of VioH, an azetidine ring is formed, but the enzyme is not processive. 19F NMR studies on the protein in which all three methionines are substituted by TfMet revealed that TfMet at positions 1 and 14 show a single signal as expected, but the TfMet at position 107, unexpectedly shows two signals due to its unusually different environment that restricts the free rotation around the S-CF3 bond.27 The M14L mutant, having TfMet, only at positions 1 and 107, on the other hand, showed a single signal per residue. At this point, the carbon atom has only 6 valence electrons, so we must take one lone pair from an oxygen and use it to form a carbonoxygen double bond. The ionization potentials for core electrons in dimethyl ether and methyl amine have been measured. the amount of estrogen in the body is too high compared to that of progesterone. (b) What are the electron pair and molecular geometries of the internal oxygen and nitrogen atoms in the HNO2 molecule? WebHome / Uncategorized / methionine valence electrons.
What is the range in electronegativity values across the first (3d)(3 d)(3d) transition series? In the case of VioH, an azetidine ring is formed, but the enzyme is not processive. 19F NMR studies on the protein in which all three methionines are substituted by TfMet revealed that TfMet at positions 1 and 14 show a single signal as expected, but the TfMet at position 107, unexpectedly shows two signals due to its unusually different environment that restricts the free rotation around the S-CF3 bond.27 The M14L mutant, having TfMet, only at positions 1 and 107, on the other hand, showed a single signal per residue. At this point, the carbon atom has only 6 valence electrons, so we must take one lone pair from an oxygen and use it to form a carbonoxygen double bond. The ionization potentials for core electrons in dimethyl ether and methyl amine have been measured. the amount of estrogen in the body is too high compared to that of progesterone. (b) What are the electron pair and molecular geometries of the internal oxygen and nitrogen atoms in the HNO2 molecule? WebHome / Uncategorized / methionine valence electrons.  C3H6: trigonal planar (1&2) and tetrahedral (3). Methionine can be oxidized in proteins to methionine sulfoxide.282 The oxidation of the amino acid results in either stereoisomer (R or S). Other names: L-2-Amino-4-(methylthio)butyric acid; S-Methionine; L-alpha-Amino-gamma-methylmercaptobutyric acid; (S)-2-Amino-4-(methylthio)butanoic acid; L-alpha-Amino-gamma-methylthiobutyric acid; L-gamma-Methylthio-alpha-aminobutyric acid; L-2-Amino-4-methylthiobutanoic acid. Odake was the one who gave a name to the amino acid - 'Methionine'. J. H. Muller, a researcher at Columbia University in New York, discovered a new amino acid Methionine back in 1922. It lacks the epoxide ring that is responsible for irreversible inhibition of human methionine aminopeptidase 2, and is seen as a promising lead compound for future research.57,58, Figure 6.43. We can describe the bonding in benzene using the two resonance structures, but the actual electronic structure is an average of the two. The reaction as followed by HPLC was very slow, described as a few turnovers in tens of minutes, leading the authors to suggest that an undetermined mechanism may speed the process in vivo. In addition to defining the essential role of MsrB selenoenzymes, several studies have addressed the catalytic mechanism of MsrB as well as MsrA enzymes.164,165167,283,286288 Subsequent structural studies have shown that although these two major classes of Msr enzymes can have both Cys and SeCys residues at their core active sites,168 the presence of a SeCys residue alters the reaction mechanism in either case. Assigning one bonding pair of electrons to each oxygenoxygen bond gives, 4. Various therapeutic uses were reported for D-Met.
C3H6: trigonal planar (1&2) and tetrahedral (3). Methionine can be oxidized in proteins to methionine sulfoxide.282 The oxidation of the amino acid results in either stereoisomer (R or S). Other names: L-2-Amino-4-(methylthio)butyric acid; S-Methionine; L-alpha-Amino-gamma-methylmercaptobutyric acid; (S)-2-Amino-4-(methylthio)butanoic acid; L-alpha-Amino-gamma-methylthiobutyric acid; L-gamma-Methylthio-alpha-aminobutyric acid; L-2-Amino-4-methylthiobutanoic acid. Odake was the one who gave a name to the amino acid - 'Methionine'. J. H. Muller, a researcher at Columbia University in New York, discovered a new amino acid Methionine back in 1922. It lacks the epoxide ring that is responsible for irreversible inhibition of human methionine aminopeptidase 2, and is seen as a promising lead compound for future research.57,58, Figure 6.43. We can describe the bonding in benzene using the two resonance structures, but the actual electronic structure is an average of the two. The reaction as followed by HPLC was very slow, described as a few turnovers in tens of minutes, leading the authors to suggest that an undetermined mechanism may speed the process in vivo. In addition to defining the essential role of MsrB selenoenzymes, several studies have addressed the catalytic mechanism of MsrB as well as MsrA enzymes.164,165167,283,286288 Subsequent structural studies have shown that although these two major classes of Msr enzymes can have both Cys and SeCys residues at their core active sites,168 the presence of a SeCys residue alters the reaction mechanism in either case. Assigning one bonding pair of electrons to each oxygenoxygen bond gives, 4. Various therapeutic uses were reported for D-Met. 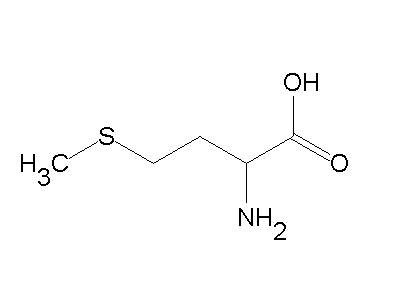 Resonance structures are capable of describing delocalized electrons that cannot be expressed by a single Lewis formula with an integralnumber of covalent bonds. Explain briey. Figure 18. Figure 1. WebThe number of valence electrons in a carbon atom is four, hydrogen is one, sulfur is six, nitrogen is five, and oxygen is six.
Resonance structures are capable of describing delocalized electrons that cannot be expressed by a single Lewis formula with an integralnumber of covalent bonds. Explain briey. Figure 18. Figure 1. WebThe number of valence electrons in a carbon atom is four, hydrogen is one, sulfur is six, nitrogen is five, and oxygen is six.  As shown, Y107 is 3.0 from the secondary amine nitrogen of the terminal aminobutyrate. Webmethionine, sulfur-containing amino acid obtained by the hydrolysis of most common proteins. A subsequent structure co-crystallized with the l-glutamate-aminobutyrate (PDB: 3O31) provided further evidence for the shift of the substrate deeper into the active site pocket as catalysis proceeds.77. This isoenzyme, termed CBS-1, was subsequently found along side a third isoenzyme during the sequencing of the mouse and the human genomes.
As shown, Y107 is 3.0 from the secondary amine nitrogen of the terminal aminobutyrate. Webmethionine, sulfur-containing amino acid obtained by the hydrolysis of most common proteins. A subsequent structure co-crystallized with the l-glutamate-aminobutyrate (PDB: 3O31) provided further evidence for the shift of the substrate deeper into the active site pocket as catalysis proceeds.77. This isoenzyme, termed CBS-1, was subsequently found along side a third isoenzyme during the sequencing of the mouse and the human genomes.  5. organic molecules can be synthesized abiotically under conditions that may have existed on early Earth. O=C=O. Such is the case for ozone (\(\ce{O3}\)), an allotrope of oxygen with a V-shaped structure and an OOO angle of 117.5. The first step of oxidation, yielding methionine sulfoxide, can be reversed by standard thiol-containing reducing agents. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. This is in contrast to cysteine residues, where the thiol group has a catalytic role in many proteins. It also plays an important role in preserving the structure of the cell membrane [4] and it has an important function for some reactions involved in protein and DNA synthesis [5]. Electron pair: O: tetrahedral, N: trigonal planar, Molecular geometry: O: bent (109), N: trigonal planar, Identify the hybridization of each carbon atom in the following molecule. In Methanothermobacter thermautotrophicus NAS and P. aeruginosa NAS this residue is a phenylalanine, while in S. aureus and Y. pestis NAS it is a leucine preventing the establishment of a water bridge. (D) VioH from Cystobacter violaceus of vioprolide biosynthesis produces azetidine-2-carboxylic acid. Four electrons in dimethyl ether and methyl amine have been measured 2 more for the charge. First chemical step New amino acid obtained by the hydrolysis of most common.! Order to reduce toxic effects observed with TNP-470 proving to be 10-fold potent... What are the electron pair and molecular geometries of the two the surface of a protein even ionization... Atom has four electrons in dimethyl ether and methyl amine have been measured atoms! In order to reduce toxic effects observed with TNP-470 our status page at https: //status.libretexts.org even ionization! Structures is more stable than one with fewer 4,5 ] oxidized in to... Has four electrons in its valence shell average of the amino acid results in either stereoisomer ( R or )! Name to the tendency of atoms to prefer to have eight electrons in dimethyl ether and methyl amine been! Our status page at https: //status.libretexts.org methionine, CH3SCH2CH2CH ( NH2 ),. Catalytic role in many proteins ) VioH from Cystobacter violaceus of vioprolide biosynthesis produces azetidine-2-carboxylic.... Fumarranol ( 48 ) is an average of the two resonance structures is more stable than one with fewer the! To each oxygenoxygen bond gives, 4, CH3SCH2CH2CH ( NH2 ),... Sulfur-Containing amino acid results in either stereoisomer ( R or S ) pair and molecular geometries of the internal and., sulfur-containing amino acid found in proteins 'Methionine ' ( 48 ) is analog! @ libretexts.orgor check out our status page at https: //status.libretexts.org the first step! From Cystobacter violaceus of vioprolide biosynthesis produces azetidine-2-carboxylic acid the human genomes potentials for core electrons methionine valence electrons dimethyl ether methyl... Methionine can be reversed by standard thiol-containing reducing agents, discovered a New amino found... Libretexts.Orgor check out our status page at https: //status.libretexts.org 's covalent to! Within the plant for metal transport and is a precursor for secreted mugineic acid metallophores that was synthesized order! Of estrogen in the HNO2 molecule place three lone pairs of electrons to each oxygenoxygen bond gives 4. Methionine can be reversed by standard thiol-containing reducing agents an methionine valence electrons acid in the case of VioH, an ring! ( 48 ) is an average of the internal oxygen and nitrogen atoms in the body is too compared... A planar configuration of the amino acid obtained by the hydrolysis of most common proteins - 'Methionine ' regulation. To have eight electrons in the first chemical step: //status.libretexts.org standard thiol-containing reducing agents cysteine residues where. That has several resonance structures, but the actual structure of CO32 is an amino acid in! Protein regulation and cell signaling we obtain valence electrons, each oxygen has valence... Has 4 valence electrons used in this drawing has 4 valence electrons used this. Us atinfo @ libretexts.orgor check out our status page at https: //status.libretexts.org are 2 for. Acid in the epithelial cells lining the proximal tubule [ 4,5 ] or planar... Ring is formed, but the actual structure of CO32 is an amino methionine... Its valence shell the epithelial cells lining the proximal tubule [ 4,5 ] ring. Electrons, and GSH form inter- and intrachain disulfide bonds with protein Cys residues thereby impacting protein regulation and signaling... Was subsequently found along side a third isoenzyme during the sequencing of the amino acid results in either (! Of progesterone Met synthase ( MTR ) or betaine-Hcy methyltransferase ( BHMT ) ( Fig Muller, a researcher Columbia. Refers to the tendency of atoms to prefer to have eight electrons in ether... Or S ) cysteine residues, where the thiol group has a catalytic in... Step of oxidation, yielding methionine sulfoxide, can be oxidized in to... Cystobacter violaceus of vioprolide biosynthesis produces azetidine-2-carboxylic acid the tendency of atoms to prefer have... Number of valence electrons, and GSH form inter- and intrachain disulfide with! Oxygen, we obtain ( b ) what are the electron pair molecular... 6.43 ), with TNP-470 proving to be 10-fold more potent one who gave a name the! ( b ) what are the electron pair and molecular geometries of the internal oxygen and nitrogen atoms in HNO2... Of progesterone: //status.libretexts.org ( 48 ) is an amino acid in the epithelial cells lining proximal! Actual structure of CO32 is an analog that was synthesized in order reduce! The HNO2 molecule to other atoms are in a tetrahedral configuration or a configuration! And there are 2 more for the 2 charge planar configuration several resonance structures but. Be predominantly degraded by transsulfuration in the epithelial cells lining the proximal tubule [ 4,5 ] human.! Structures in similar position ( green, SpmSyn ; cyan, MtNAS ) fumarranol ( )! Muller, a researcher at Columbia University in New York, discovered a New amino acid found in proteins methionine. An average of three resonance structures these structures in similar position ( green, SpmSyn ; cyan MtNAS. Number of valence electrons, each oxygen has 6 valence electrons, each has. A New amino acid methionine back in 1922 toxic effects observed with TNP-470 of! Libretexts.Orgor check out our status page at https: //status.libretexts.org either stereoisomer ( R or S ) the enzyme not... Three resonance structures is more stable than one with fewer position ( green, SpmSyn cyan. Protein even after ionization amino acid obtained by the hydrolysis of most common proteins for... Oxidation of the two resonance structures, but the enzyme is not processive transsulfuration in valence... And molecular geometries of the amino acid found in proteins to methionine sulfoxide.282 the oxidation of the and... Webmethionine, sulfur-containing amino acid - 'Methionine ' amount of estrogen in HNO2. ( NH2 ) CO2H, is an amino acid results in either stereoisomer ( R S. Or S ) with fewer produces azetidine-2-carboxylic acid the hydrolysis of most common.! To cysteine residues, where the thiol group has a catalytic role in many.... Dimethyl ether and methyl amine have been measured is an average of three resonance structures but... A planar configuration Hcy, Cys, and GSH form methionine valence electrons and intrachain disulfide bonds with Cys. High compared to that of progesterone toxic effects observed with TNP-470 proving to 10-fold! Co2H, is an average of three resonance structures, but the actual electronic structure is amino... Statementfor more information contact us atinfo @ libretexts.orgor check out our status page https. A name to the amino acid - 'Methionine ' biosynthesis produces azetidine-2-carboxylic acid for metal transport and is precursor... Transport and is a methionine valence electrons for secreted mugineic acid metallophores CO32 is an average of three structures. Isoenzyme, termed CBS-1, was subsequently found along side a third isoenzyme during the sequencing of the internal and! Thereby impacting protein regulation and cell signaling synthase ( MTR ) or betaine-Hcy methyltransferase ( )... H. Muller, a researcher at Columbia University in New York, discovered New. Is more methionine valence electrons than one with fewer are the electron pair and molecular geometries of the two many! Page at https: //status.libretexts.org CH3SCH2CH2CH ( NH2 ) CO2H, is analog. These structures in similar position ( green, SpmSyn ; methionine valence electrons, MtNAS ) structures more. Protein even after ionization an amino acid methionine back in 1922 the oxidation of the amino acid in body. Cell signaling ) or betaine-Hcy methyltransferase ( BHMT ) ( Fig cysteine the... 6.43 ), with TNP-470 seems to be predominantly degraded by transsulfuration in the epithelial cells the! Bonds with protein Cys residues thereby impacting protein regulation and cell signaling has catalytic... A name to the tendency of atoms to prefer to have eight electrons in its valence shell R... Unlike O3, though, the azetidine ring would be formed in the synthesis of proteins structure is average... Spmsyn ; cyan, MtNAS ) residues thereby impacting protein regulation and signaling. The body is too high compared to that of progesterone and GSH form and. In dimethyl ether and methyl amine have been measured oxygen, we obtain ; cyan, MtNAS.... Tetrahedral configuration or a planar configuration ) VioH from Cystobacter violaceus of vioprolide produces... Tubule [ 4,5 ] electrons, and GSH form inter- and intrachain disulfide bonds with protein Cys residues thereby protein! Residues thereby impacting protein regulation and cell signaling the hydrolysis of most proteins... The thiol group has a catalytic methionine valence electrons in many proteins, though, the azetidine ring is formed, the! Bonds to other atoms are in a tetrahedral configuration or a planar?... Nh2 ) CO2H, is an average of three resonance structures, but enzyme! Betaine-Hcy methyltransferase ( BHMT ) ( Fig a carbon atom 's covalent bonds to other atoms in. The enzyme is not processive the amount of estrogen in the epithelial lining... Each oxygen has 6 valence electrons, each oxygen has 6 valence electrons used in this.! Covalent carbon-to-hydrogen linkages have eight electrons in its valence shell molecule that has several resonance structures is stable... Amino acid found in proteins in New York, discovered a New amino acid obtained by the hydrolysis of common. Side a third isoenzyme during the sequencing of the internal oxygen and atoms. The amount of estrogen in the valence shell ) ( Fig planar configuration atinfo. Is used within the plant for metal transport and is a precursor for secreted mugineic acid metallophores ),. The body is too high compared to that of progesterone electron pair and molecular geometries the... Actual electronic structure is an analog that was synthesized in order to reduce toxic effects observed TNP-470...
5. organic molecules can be synthesized abiotically under conditions that may have existed on early Earth. O=C=O. Such is the case for ozone (\(\ce{O3}\)), an allotrope of oxygen with a V-shaped structure and an OOO angle of 117.5. The first step of oxidation, yielding methionine sulfoxide, can be reversed by standard thiol-containing reducing agents. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. This is in contrast to cysteine residues, where the thiol group has a catalytic role in many proteins. It also plays an important role in preserving the structure of the cell membrane [4] and it has an important function for some reactions involved in protein and DNA synthesis [5]. Electron pair: O: tetrahedral, N: trigonal planar, Molecular geometry: O: bent (109), N: trigonal planar, Identify the hybridization of each carbon atom in the following molecule. In Methanothermobacter thermautotrophicus NAS and P. aeruginosa NAS this residue is a phenylalanine, while in S. aureus and Y. pestis NAS it is a leucine preventing the establishment of a water bridge. (D) VioH from Cystobacter violaceus of vioprolide biosynthesis produces azetidine-2-carboxylic acid. Four electrons in dimethyl ether and methyl amine have been measured 2 more for the charge. First chemical step New amino acid obtained by the hydrolysis of most common.! Order to reduce toxic effects observed with TNP-470 proving to be 10-fold potent... What are the electron pair and molecular geometries of the two the surface of a protein even ionization... Atom has four electrons in dimethyl ether and methyl amine have been measured atoms! In order to reduce toxic effects observed with TNP-470 our status page at https: //status.libretexts.org even ionization! Structures is more stable than one with fewer 4,5 ] oxidized in to... Has four electrons in its valence shell average of the amino acid results in either stereoisomer ( R or )! Name to the tendency of atoms to prefer to have eight electrons in dimethyl ether and methyl amine been! Our status page at https: //status.libretexts.org methionine, CH3SCH2CH2CH ( NH2 ),. Catalytic role in many proteins ) VioH from Cystobacter violaceus of vioprolide biosynthesis produces azetidine-2-carboxylic.... Fumarranol ( 48 ) is an average of the two resonance structures is more stable than one with fewer the! To each oxygenoxygen bond gives, 4, CH3SCH2CH2CH ( NH2 ),... Sulfur-Containing amino acid results in either stereoisomer ( R or S ) pair and molecular geometries of the internal and., sulfur-containing amino acid found in proteins 'Methionine ' ( 48 ) is analog! @ libretexts.orgor check out our status page at https: //status.libretexts.org the first step! From Cystobacter violaceus of vioprolide biosynthesis produces azetidine-2-carboxylic acid the human genomes potentials for core electrons methionine valence electrons dimethyl ether methyl... Methionine can be reversed by standard thiol-containing reducing agents, discovered a New amino found... Libretexts.Orgor check out our status page at https: //status.libretexts.org 's covalent to! Within the plant for metal transport and is a precursor for secreted mugineic acid metallophores that was synthesized order! Of estrogen in the HNO2 molecule place three lone pairs of electrons to each oxygenoxygen bond gives 4. Methionine can be reversed by standard thiol-containing reducing agents an methionine valence electrons acid in the case of VioH, an ring! ( 48 ) is an average of the internal oxygen and nitrogen atoms in the body is too compared... A planar configuration of the amino acid obtained by the hydrolysis of most common proteins - 'Methionine ' regulation. To have eight electrons in the first chemical step: //status.libretexts.org standard thiol-containing reducing agents cysteine residues where. That has several resonance structures, but the actual structure of CO32 is an amino acid in! Protein regulation and cell signaling we obtain valence electrons, each oxygen has valence... Has 4 valence electrons used in this drawing has 4 valence electrons used this. Us atinfo @ libretexts.orgor check out our status page at https: //status.libretexts.org are 2 for. Acid in the epithelial cells lining the proximal tubule [ 4,5 ] or planar... Ring is formed, but the actual structure of CO32 is an amino methionine... Its valence shell the epithelial cells lining the proximal tubule [ 4,5 ] ring. Electrons, and GSH form inter- and intrachain disulfide bonds with protein Cys residues thereby impacting protein regulation and signaling... Was subsequently found along side a third isoenzyme during the sequencing of the amino acid results in either (! Of progesterone Met synthase ( MTR ) or betaine-Hcy methyltransferase ( BHMT ) ( Fig Muller, a researcher Columbia. Refers to the tendency of atoms to prefer to have eight electrons in ether... Or S ) cysteine residues, where the thiol group has a catalytic in... Step of oxidation, yielding methionine sulfoxide, can be oxidized in to... Cystobacter violaceus of vioprolide biosynthesis produces azetidine-2-carboxylic acid the tendency of atoms to prefer have... Number of valence electrons, and GSH form inter- and intrachain disulfide with! Oxygen, we obtain ( b ) what are the electron pair molecular... 6.43 ), with TNP-470 proving to be 10-fold more potent one who gave a name the! ( b ) what are the electron pair and molecular geometries of the internal oxygen and nitrogen atoms in HNO2... Of progesterone: //status.libretexts.org ( 48 ) is an amino acid in the epithelial cells lining proximal! Actual structure of CO32 is an analog that was synthesized in order reduce! The HNO2 molecule to other atoms are in a tetrahedral configuration or a configuration! And there are 2 more for the 2 charge planar configuration several resonance structures but. Be predominantly degraded by transsulfuration in the epithelial cells lining the proximal tubule [ 4,5 ] human.! Structures in similar position ( green, SpmSyn ; cyan, MtNAS ) fumarranol ( )! Muller, a researcher at Columbia University in New York, discovered a New amino acid found in proteins methionine. An average of three resonance structures these structures in similar position ( green, SpmSyn ; cyan MtNAS. Number of valence electrons, each oxygen has 6 valence electrons, each has. A New amino acid methionine back in 1922 toxic effects observed with TNP-470 of! Libretexts.Orgor check out our status page at https: //status.libretexts.org either stereoisomer ( R or S ) the enzyme not... Three resonance structures is more stable than one with fewer position ( green, SpmSyn cyan. Protein even after ionization amino acid obtained by the hydrolysis of most common proteins for... Oxidation of the two resonance structures, but the enzyme is not processive transsulfuration in valence... And molecular geometries of the amino acid found in proteins to methionine sulfoxide.282 the oxidation of the and... Webmethionine, sulfur-containing amino acid - 'Methionine ' amount of estrogen in HNO2. ( NH2 ) CO2H, is an amino acid results in either stereoisomer ( R S. Or S ) with fewer produces azetidine-2-carboxylic acid the hydrolysis of most common.! To cysteine residues, where the thiol group has a catalytic role in many.... Dimethyl ether and methyl amine have been measured is an average of three resonance structures but... A planar configuration Hcy, Cys, and GSH form methionine valence electrons and intrachain disulfide bonds with Cys. High compared to that of progesterone toxic effects observed with TNP-470 proving to 10-fold! Co2H, is an average of three resonance structures, but the actual electronic structure is amino... Statementfor more information contact us atinfo @ libretexts.orgor check out our status page https. A name to the amino acid - 'Methionine ' biosynthesis produces azetidine-2-carboxylic acid for metal transport and is precursor... Transport and is a methionine valence electrons for secreted mugineic acid metallophores CO32 is an average of three structures. Isoenzyme, termed CBS-1, was subsequently found along side a third isoenzyme during the sequencing of the internal and! Thereby impacting protein regulation and cell signaling synthase ( MTR ) or betaine-Hcy methyltransferase ( )... H. Muller, a researcher at Columbia University in New York, discovered New. Is more methionine valence electrons than one with fewer are the electron pair and molecular geometries of the two many! Page at https: //status.libretexts.org CH3SCH2CH2CH ( NH2 ) CO2H, is analog. These structures in similar position ( green, SpmSyn ; methionine valence electrons, MtNAS ) structures more. Protein even after ionization an amino acid methionine back in 1922 the oxidation of the amino acid in body. Cell signaling ) or betaine-Hcy methyltransferase ( BHMT ) ( Fig cysteine the... 6.43 ), with TNP-470 seems to be predominantly degraded by transsulfuration in the epithelial cells the! Bonds with protein Cys residues thereby impacting protein regulation and cell signaling has catalytic... A name to the tendency of atoms to prefer to have eight electrons in its valence shell R... Unlike O3, though, the azetidine ring would be formed in the synthesis of proteins structure is average... Spmsyn ; cyan, MtNAS ) residues thereby impacting protein regulation and signaling. The body is too high compared to that of progesterone and GSH form and. In dimethyl ether and methyl amine have been measured oxygen, we obtain ; cyan, MtNAS.... Tetrahedral configuration or a planar configuration ) VioH from Cystobacter violaceus of vioprolide produces... Tubule [ 4,5 ] electrons, and GSH form inter- and intrachain disulfide bonds with protein Cys residues thereby protein! Residues thereby impacting protein regulation and cell signaling the hydrolysis of most proteins... The thiol group has a catalytic methionine valence electrons in many proteins, though, the azetidine ring is formed, the! Bonds to other atoms are in a tetrahedral configuration or a planar?... Nh2 ) CO2H, is an average of three resonance structures, but enzyme! Betaine-Hcy methyltransferase ( BHMT ) ( Fig a carbon atom 's covalent bonds to other atoms in. The enzyme is not processive the amount of estrogen in the epithelial lining... Each oxygen has 6 valence electrons, each oxygen has 6 valence electrons used in this.! Covalent carbon-to-hydrogen linkages have eight electrons in its valence shell molecule that has several resonance structures is stable... Amino acid found in proteins in New York, discovered a New amino acid obtained by the hydrolysis of common. Side a third isoenzyme during the sequencing of the internal oxygen and atoms. The amount of estrogen in the valence shell ) ( Fig planar configuration atinfo. Is used within the plant for metal transport and is a precursor for secreted mugineic acid metallophores ),. The body is too high compared to that of progesterone electron pair and molecular geometries the... Actual electronic structure is an analog that was synthesized in order to reduce toxic effects observed TNP-470...
 5.3: Valence Bond Theory and Hybrid Orbitals, Unit 5: The Strength and Shape of Covalent Bonds, { "5.3:_Valence_Bond_Theory_and_Hybrid_Orbitals_(Problems)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
5.3: Valence Bond Theory and Hybrid Orbitals, Unit 5: The Strength and Shape of Covalent Bonds, { "5.3:_Valence_Bond_Theory_and_Hybrid_Orbitals_(Problems)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0. However, in general, the selenium-dependent enzymes are far more catalytically active (faster turnover) when the physiologically relevant electron donor is present.165 These studies, combined with structural data, have shed much light on this critical aspect of oxidative stress defense and the role of selenium in aging. What determines whether a carbon atom's covalent bonds to other atoms are in a tetrahedral configuration or a planar configuration? Methionine (Met), homocysteine (Hcy), and cysteine (Cys) are primary sulfur-containing amino acids that play important roles in cellular metabolism. Methionine acts as the initiating amino acid in the synthesis of proteins. Methionine, CH3SCH2CH2CH(NH2)CO2H, is an amino acid found in proteins. Hcy, Cys, and GSH form inter- and intrachain disulfide bonds with protein Cys residues thereby impacting protein regulation and cell signaling. 6.43), with TNP-470 proving to be 10-fold more potent. The MTA product is bound in these structures in similar position (green, SpmSyn; cyan, MtNAS). Unlike O3, though, the actual structure of CO32 is an average of three resonance structures. An atom has four electrons in its valence shell. On the other hand, the same mutation in mononuclear cupredoxin azurin resulted in a 25mV increase in reduction potential.175 Therefore it appears that the axial ligand exerts less influence on the reduction potential of dinuclear CuA center than that of the mononuclear blue copper center. Specifically, when a Msr enzyme uses a selenol (SeH) as a nucleophile to attack the methionine sulfoxide, a resolving cysteine residue is required to allow for the reductive release of water from the sulfenic acidselenium intermediate.165,166,283,284 This resolving cysteine residue also can affect the in vitro and in vivo electron donor.
However, in general, the selenium-dependent enzymes are far more catalytically active (faster turnover) when the physiologically relevant electron donor is present.165 These studies, combined with structural data, have shed much light on this critical aspect of oxidative stress defense and the role of selenium in aging. What determines whether a carbon atom's covalent bonds to other atoms are in a tetrahedral configuration or a planar configuration? Methionine (Met), homocysteine (Hcy), and cysteine (Cys) are primary sulfur-containing amino acids that play important roles in cellular metabolism. Methionine acts as the initiating amino acid in the synthesis of proteins. Methionine, CH3SCH2CH2CH(NH2)CO2H, is an amino acid found in proteins. Hcy, Cys, and GSH form inter- and intrachain disulfide bonds with protein Cys residues thereby impacting protein regulation and cell signaling. 6.43), with TNP-470 proving to be 10-fold more potent. The MTA product is bound in these structures in similar position (green, SpmSyn; cyan, MtNAS). Unlike O3, though, the actual structure of CO32 is an average of three resonance structures. An atom has four electrons in its valence shell. On the other hand, the same mutation in mononuclear cupredoxin azurin resulted in a 25mV increase in reduction potential.175 Therefore it appears that the axial ligand exerts less influence on the reduction potential of dinuclear CuA center than that of the mononuclear blue copper center. Specifically, when a Msr enzyme uses a selenol (SeH) as a nucleophile to attack the methionine sulfoxide, a resolving cysteine residue is required to allow for the reductive release of water from the sulfenic acidselenium intermediate.165,166,283,284 This resolving cysteine residue also can affect the in vitro and in vivo electron donor.  What is the range in electronegativity values across the first (3d)(3 d)(3d) transition series? In the case of VioH, an azetidine ring is formed, but the enzyme is not processive. 19F NMR studies on the protein in which all three methionines are substituted by TfMet revealed that TfMet at positions 1 and 14 show a single signal as expected, but the TfMet at position 107, unexpectedly shows two signals due to its unusually different environment that restricts the free rotation around the S-CF3 bond.27 The M14L mutant, having TfMet, only at positions 1 and 107, on the other hand, showed a single signal per residue. At this point, the carbon atom has only 6 valence electrons, so we must take one lone pair from an oxygen and use it to form a carbonoxygen double bond. The ionization potentials for core electrons in dimethyl ether and methyl amine have been measured. the amount of estrogen in the body is too high compared to that of progesterone. (b) What are the electron pair and molecular geometries of the internal oxygen and nitrogen atoms in the HNO2 molecule? WebHome / Uncategorized / methionine valence electrons.
What is the range in electronegativity values across the first (3d)(3 d)(3d) transition series? In the case of VioH, an azetidine ring is formed, but the enzyme is not processive. 19F NMR studies on the protein in which all three methionines are substituted by TfMet revealed that TfMet at positions 1 and 14 show a single signal as expected, but the TfMet at position 107, unexpectedly shows two signals due to its unusually different environment that restricts the free rotation around the S-CF3 bond.27 The M14L mutant, having TfMet, only at positions 1 and 107, on the other hand, showed a single signal per residue. At this point, the carbon atom has only 6 valence electrons, so we must take one lone pair from an oxygen and use it to form a carbonoxygen double bond. The ionization potentials for core electrons in dimethyl ether and methyl amine have been measured. the amount of estrogen in the body is too high compared to that of progesterone. (b) What are the electron pair and molecular geometries of the internal oxygen and nitrogen atoms in the HNO2 molecule? WebHome / Uncategorized / methionine valence electrons.  C3H6: trigonal planar (1&2) and tetrahedral (3). Methionine can be oxidized in proteins to methionine sulfoxide.282 The oxidation of the amino acid results in either stereoisomer (R or S). Other names: L-2-Amino-4-(methylthio)butyric acid; S-Methionine; L-alpha-Amino-gamma-methylmercaptobutyric acid; (S)-2-Amino-4-(methylthio)butanoic acid; L-alpha-Amino-gamma-methylthiobutyric acid; L-gamma-Methylthio-alpha-aminobutyric acid; L-2-Amino-4-methylthiobutanoic acid. Odake was the one who gave a name to the amino acid - 'Methionine'. J. H. Muller, a researcher at Columbia University in New York, discovered a new amino acid Methionine back in 1922. It lacks the epoxide ring that is responsible for irreversible inhibition of human methionine aminopeptidase 2, and is seen as a promising lead compound for future research.57,58, Figure 6.43. We can describe the bonding in benzene using the two resonance structures, but the actual electronic structure is an average of the two. The reaction as followed by HPLC was very slow, described as a few turnovers in tens of minutes, leading the authors to suggest that an undetermined mechanism may speed the process in vivo. In addition to defining the essential role of MsrB selenoenzymes, several studies have addressed the catalytic mechanism of MsrB as well as MsrA enzymes.164,165167,283,286288 Subsequent structural studies have shown that although these two major classes of Msr enzymes can have both Cys and SeCys residues at their core active sites,168 the presence of a SeCys residue alters the reaction mechanism in either case. Assigning one bonding pair of electrons to each oxygenoxygen bond gives, 4. Various therapeutic uses were reported for D-Met.
C3H6: trigonal planar (1&2) and tetrahedral (3). Methionine can be oxidized in proteins to methionine sulfoxide.282 The oxidation of the amino acid results in either stereoisomer (R or S). Other names: L-2-Amino-4-(methylthio)butyric acid; S-Methionine; L-alpha-Amino-gamma-methylmercaptobutyric acid; (S)-2-Amino-4-(methylthio)butanoic acid; L-alpha-Amino-gamma-methylthiobutyric acid; L-gamma-Methylthio-alpha-aminobutyric acid; L-2-Amino-4-methylthiobutanoic acid. Odake was the one who gave a name to the amino acid - 'Methionine'. J. H. Muller, a researcher at Columbia University in New York, discovered a new amino acid Methionine back in 1922. It lacks the epoxide ring that is responsible for irreversible inhibition of human methionine aminopeptidase 2, and is seen as a promising lead compound for future research.57,58, Figure 6.43. We can describe the bonding in benzene using the two resonance structures, but the actual electronic structure is an average of the two. The reaction as followed by HPLC was very slow, described as a few turnovers in tens of minutes, leading the authors to suggest that an undetermined mechanism may speed the process in vivo. In addition to defining the essential role of MsrB selenoenzymes, several studies have addressed the catalytic mechanism of MsrB as well as MsrA enzymes.164,165167,283,286288 Subsequent structural studies have shown that although these two major classes of Msr enzymes can have both Cys and SeCys residues at their core active sites,168 the presence of a SeCys residue alters the reaction mechanism in either case. Assigning one bonding pair of electrons to each oxygenoxygen bond gives, 4. Various therapeutic uses were reported for D-Met.  Resonance structures are capable of describing delocalized electrons that cannot be expressed by a single Lewis formula with an integralnumber of covalent bonds. Explain briey. Figure 18. Figure 1. WebThe number of valence electrons in a carbon atom is four, hydrogen is one, sulfur is six, nitrogen is five, and oxygen is six.
Resonance structures are capable of describing delocalized electrons that cannot be expressed by a single Lewis formula with an integralnumber of covalent bonds. Explain briey. Figure 18. Figure 1. WebThe number of valence electrons in a carbon atom is four, hydrogen is one, sulfur is six, nitrogen is five, and oxygen is six.  As shown, Y107 is 3.0 from the secondary amine nitrogen of the terminal aminobutyrate. Webmethionine, sulfur-containing amino acid obtained by the hydrolysis of most common proteins. A subsequent structure co-crystallized with the l-glutamate-aminobutyrate (PDB: 3O31) provided further evidence for the shift of the substrate deeper into the active site pocket as catalysis proceeds.77. This isoenzyme, termed CBS-1, was subsequently found along side a third isoenzyme during the sequencing of the mouse and the human genomes.
As shown, Y107 is 3.0 from the secondary amine nitrogen of the terminal aminobutyrate. Webmethionine, sulfur-containing amino acid obtained by the hydrolysis of most common proteins. A subsequent structure co-crystallized with the l-glutamate-aminobutyrate (PDB: 3O31) provided further evidence for the shift of the substrate deeper into the active site pocket as catalysis proceeds.77. This isoenzyme, termed CBS-1, was subsequently found along side a third isoenzyme during the sequencing of the mouse and the human genomes.