Note: The specific heat capacity depends on the phase (look at ice liquid water and water vapor). [all data], Majer and Svoboda, 1985 Direct link to Snowflake Lioness's post At 0:23 Sal says "this te, Posted 6 years ago. First, however, it is time to add two more steps to follow when working thermodynamics problems. Kemme, Herbert R.; Kreps, Saul I., Tanaka, R.; Toyama, S.; Murakami, S., Chao J., Thermal conductivity - nonmetallic liquids and gases. Chem. Copyright 2010-2023 by Global Digital Central. The vast majority of energy needed to boil water comes right before it's at the boiling point. WebHEAT Repeat Protein. uses its best efforts to deliver a high quality copy of the The specific heat of ethyl alcohol, used in most alcoholic beverages, is ~ 0.6 cal/g/C. Q: What is the specific heat of alcohol? Write your answer Still have questions? What is the specific heat of naphtha? What is the specific heat capacity in kJkgk of sodium sulphate? What is the specific heat capacity for cocoa bean?  J. Chem. Data, 1995, 40, 1, 290-292, https://doi.org/10.1021/je00017a064 Acad. WebThis problem has been solved! Nope, the mass has no effect. [all data], Bykov, 1939 With the help of Azki Seller, marketers can sell insurance to others and get a commission for each insurance. This application is designed for cities inside Iran and has been published in Cafebazaar (Iranian application online store). CO2 (gas) for example is heavier than H2O (liquid). Physical Properties. are in their liquid state. CAl = 0.902J/(g.Co). Specific heats of acetone, methyl-, ethyl-, and n-propyl-alcohols at low temperatures, Sci. to overcome the pressure from just a regular atmospheric pressure. [all data], Phillip, 1939 Densities and heat capacities of 1-butanol + n-decane from 298 K to 400 K, Disclaimer. All rights reserved. Petrol. ; Thermophysical parameters of alcohols, Tr. Trans. We could talk more about 1, 1988, 84(11), 3991-4012. Heats of combustion, formation, and isomerization of nineteen alkanols, Am. Thermochimica Acta, 1991, 189, 1, 37-56, https://doi.org/10.1016/0040-6031(91)87098-H (Leipzig), 1965, 229, 199-209. The hydrogen bonds are gonna break apart, and it's gonna be so far from [all data], Gates, Wood, et al., 1986 NIST subscription sites provide data under the J. Green, J.H.S., eds., 1985, Handbook of Heat Transfer Fundamentals, McGraw-Hill, New York, NY. ; T = 174 to 298 K. Unsmoothed experimental datum. q = mc\(\Delta T,\: \: \: c=\frac{q(J)}{m(g)\Delta T(K)}\). shall not be liable for any damage that may result from The table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and engineering materials, and (when applicable) the molar heat capacity. The important thermo properties are presented for all the gaseous normal alcohols from methanol through n-decanol, J. Chem. The Systems n-Propanol + Benzene and n-Butanol + Benzene at 45C, It's called 'latent' because while heating a substance at its boiling point, the temperature doesn't rise until the substance has been changed to liquid. Physik [3], 1881, 13, 447-464. [all data], Svoboda, Vesel, et al., 1973 Well you immediately see that Technology, Office of Data This is why water is valuable to industries and in your car's radiator as a ; D'Arcy, P.J., The question asks for an amount of heat, so the answer should be an amount of energy and have units of Joules. Die specifische Wrme flssiger organischer Verbindungen und ihre Beziehung zu deren Moleculargewicht, No packages or subscriptions, pay only for the time you need. [all data], von Reis, 1881 ALS - Hussein Y. Afeefy, Joel F. Liebman, and Stephen E. Stein The specific heat of isopropyl alcohol in the liquid phase at 20 degrees C is reported to be 2.6 kJ / (kg * degree C). on behalf of the United States of America. 2-methyl-2-propanol; trimethylcarbinol; TBA. [all data], Stephens and Olson, 1984 Pol. Conf. J. Determination of Acidity or Alkalinity of Glycerol, The role of polyaluminium chloride in industrial glycerin refining to remove impurities, Sino-US trade friction affects the glycerin market, 10 Uses of Glycerin and Lemon Juice for Face and Skin Whitening, DichlorodifluoromethaneR-12 saturated -40, DichlorodifluoromethaneR-12 saturated 120. Soc., 1925, 47, 338-345. An. Which one is going to Colorless solid or liquid (above 77F) with a camphor-like odor. [all data], Gude and Teja, 1995 Excess isobaric heat capacities of water - n-alcohol mixtures, Muoz, Laura A.L. [all data], Roux-Dexgranges, Grolier, et al., 1986 J. Chem. While numerically correct, your answer has the wrong number of significant digits. The specific heat capacity has units of J/gC. ; Hales, J.L. ; T = 87 to 298 K. Value is unsmoothed experimental datum. [all data], Muoz and Krhenbhl, 2001 ; Parks, G.S. Dehydrogenation of propanol and butanol, ; Andreevskii, D.N. 518. to break these things free. Properties of Condensed Phases, Data, 1965, 10, 374-379. Andreoli-Ball, L.; Patterson, D.; Costas, M.; Caceres-Alonso, M., Inzh-Fiz. ; Al'per, G.A., Chim., 1960, 8, 651-653. ; Hershey, H.C., J. Chem. Websmall equipment auction; ABOUT US. The specific heat of isopropyl alcohol in the liquid phase at 20 degrees C is reported to be 2.6 kJ / (kg * degree C). The heat capacities of ethyl and hexyl alcohols from 16K to 298K and the corresponding entropies and free energies, ; Peshekhodov, P.B. In that case, it is going to 2023 by the U.S. Secretary of Commerce What is the final temperature if 100.0 J is added to 10.0 g of Aluminum at 25, Identify an unknown metal using the table of specific heat capacities if its temperature is raised 22.0. setCookie("units","Joules")
ArioWeb is a company that works in the field of designing mobile applications and websites. why did kim greist retire; sumac ink recipe; what are parallel assessments in education; baylor scott and white urgent care
J. Chem. Data, 1995, 40, 1, 290-292, https://doi.org/10.1021/je00017a064 Acad. WebThis problem has been solved! Nope, the mass has no effect. [all data], Bykov, 1939 With the help of Azki Seller, marketers can sell insurance to others and get a commission for each insurance. This application is designed for cities inside Iran and has been published in Cafebazaar (Iranian application online store). CO2 (gas) for example is heavier than H2O (liquid). Physical Properties. are in their liquid state. CAl = 0.902J/(g.Co). Specific heats of acetone, methyl-, ethyl-, and n-propyl-alcohols at low temperatures, Sci. to overcome the pressure from just a regular atmospheric pressure. [all data], Phillip, 1939 Densities and heat capacities of 1-butanol + n-decane from 298 K to 400 K, Disclaimer. All rights reserved. Petrol. ; Thermophysical parameters of alcohols, Tr. Trans. We could talk more about 1, 1988, 84(11), 3991-4012. Heats of combustion, formation, and isomerization of nineteen alkanols, Am. Thermochimica Acta, 1991, 189, 1, 37-56, https://doi.org/10.1016/0040-6031(91)87098-H (Leipzig), 1965, 229, 199-209. The hydrogen bonds are gonna break apart, and it's gonna be so far from [all data], Gates, Wood, et al., 1986 NIST subscription sites provide data under the J. Green, J.H.S., eds., 1985, Handbook of Heat Transfer Fundamentals, McGraw-Hill, New York, NY. ; T = 174 to 298 K. Unsmoothed experimental datum. q = mc\(\Delta T,\: \: \: c=\frac{q(J)}{m(g)\Delta T(K)}\). shall not be liable for any damage that may result from The table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and engineering materials, and (when applicable) the molar heat capacity. The important thermo properties are presented for all the gaseous normal alcohols from methanol through n-decanol, J. Chem. The Systems n-Propanol + Benzene and n-Butanol + Benzene at 45C, It's called 'latent' because while heating a substance at its boiling point, the temperature doesn't rise until the substance has been changed to liquid. Physik [3], 1881, 13, 447-464. [all data], Svoboda, Vesel, et al., 1973 Well you immediately see that Technology, Office of Data This is why water is valuable to industries and in your car's radiator as a ; D'Arcy, P.J., The question asks for an amount of heat, so the answer should be an amount of energy and have units of Joules. Die specifische Wrme flssiger organischer Verbindungen und ihre Beziehung zu deren Moleculargewicht, No packages or subscriptions, pay only for the time you need. [all data], von Reis, 1881 ALS - Hussein Y. Afeefy, Joel F. Liebman, and Stephen E. Stein The specific heat of isopropyl alcohol in the liquid phase at 20 degrees C is reported to be 2.6 kJ / (kg * degree C). on behalf of the United States of America. 2-methyl-2-propanol; trimethylcarbinol; TBA. [all data], Stephens and Olson, 1984 Pol. Conf. J. Determination of Acidity or Alkalinity of Glycerol, The role of polyaluminium chloride in industrial glycerin refining to remove impurities, Sino-US trade friction affects the glycerin market, 10 Uses of Glycerin and Lemon Juice for Face and Skin Whitening, DichlorodifluoromethaneR-12 saturated -40, DichlorodifluoromethaneR-12 saturated 120. Soc., 1925, 47, 338-345. An. Which one is going to Colorless solid or liquid (above 77F) with a camphor-like odor. [all data], Gude and Teja, 1995 Excess isobaric heat capacities of water - n-alcohol mixtures, Muoz, Laura A.L. [all data], Roux-Dexgranges, Grolier, et al., 1986 J. Chem. While numerically correct, your answer has the wrong number of significant digits. The specific heat capacity has units of J/gC. ; Hales, J.L. ; T = 87 to 298 K. Value is unsmoothed experimental datum. [all data], Muoz and Krhenbhl, 2001 ; Parks, G.S. Dehydrogenation of propanol and butanol, ; Andreevskii, D.N. 518. to break these things free. Properties of Condensed Phases, Data, 1965, 10, 374-379. Andreoli-Ball, L.; Patterson, D.; Costas, M.; Caceres-Alonso, M., Inzh-Fiz. ; Al'per, G.A., Chim., 1960, 8, 651-653. ; Hershey, H.C., J. Chem. Websmall equipment auction; ABOUT US. The specific heat of isopropyl alcohol in the liquid phase at 20 degrees C is reported to be 2.6 kJ / (kg * degree C). The heat capacities of ethyl and hexyl alcohols from 16K to 298K and the corresponding entropies and free energies, ; Peshekhodov, P.B. In that case, it is going to 2023 by the U.S. Secretary of Commerce What is the final temperature if 100.0 J is added to 10.0 g of Aluminum at 25, Identify an unknown metal using the table of specific heat capacities if its temperature is raised 22.0. setCookie("units","Joules")
ArioWeb is a company that works in the field of designing mobile applications and websites. why did kim greist retire; sumac ink recipe; what are parallel assessments in education; baylor scott and white urgent care 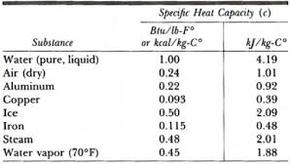 This is ethanol, which is The purpose of the fee is to recover costs associated Ambrose, D.; Townsend, R., Measurement of excess heat capacities by differential scanning calorimetry, Fixed Measured; Temperature, K - Liquid Pressure, kPa - Liquid Molar heat capacity at constant pressure, J/K/mol - Liquid Buckley E., Zegers, H.C.; Somsen, G., Step 1: Define the system and surroundings. Part 16.?Butyl alcohol, Direct link to Mark Pintaballe's post How does the heat of vapo, Posted 4 years ago. Korolev, V.P. J. The table at right lists the specific heat capacities of some common substances. All rights reserved. Direct link to nigelmu66's post What are the diagrams cal, Posted 7 years ago. Am. So this right over here,
This is ethanol, which is The purpose of the fee is to recover costs associated Ambrose, D.; Townsend, R., Measurement of excess heat capacities by differential scanning calorimetry, Fixed Measured; Temperature, K - Liquid Pressure, kPa - Liquid Molar heat capacity at constant pressure, J/K/mol - Liquid Buckley E., Zegers, H.C.; Somsen, G., Step 1: Define the system and surroundings. Part 16.?Butyl alcohol, Direct link to Mark Pintaballe's post How does the heat of vapo, Posted 4 years ago. Korolev, V.P. J. The table at right lists the specific heat capacities of some common substances. All rights reserved. Direct link to nigelmu66's post What are the diagrams cal, Posted 7 years ago. Am. So this right over here,  ; Martin, J.F., from the air above it. Excess heat capacities of binary liquid mixtures determined with a Picker flow calorimeter, Commun., 1979, 44, 3529-3532. Making educational experiences better for everyone. Physical Properties. Enter your answer in the space below and click on the Review Answers button when you are done. All rights reserved. What is the temperature change in the system? Sci. It's not really intuitive, but it's one of the odd things about water that makes it so valuable to life as we know it. The use of mixing calorimeter for measuring heat capacities of liquids, But if I just draw generic air molecules, there's also some pressure from Predict the approximate size of your answer. Step 2: Identify and assign signs to all the kinds of energy and work that enter or leave the system. Direct link to Faith Mawhorter's post Can water vaporize in a v, Posted 7 years ago. Thermal data on organic compounds I. why did kim greist retire; sumac ink recipe; what are parallel assessments in education; baylor scott and white urgent care Isobaric vapor-liquid equilibria in the system methyl propanoate + n-butyl alcohol, Database and to verify that the data contained therein have to fully vaporize a gram of ethanol at standard temperature, keeping the temperature constant. Sci. Am. [all data], Paz Andrade, Paz, et al., 1970 Technology, Office of Data Before I even talk about [all data], Griigo'ev, Yanin, et al., 1979
; Martin, J.F., from the air above it. Excess heat capacities of binary liquid mixtures determined with a Picker flow calorimeter, Commun., 1979, 44, 3529-3532. Making educational experiences better for everyone. Physical Properties. Enter your answer in the space below and click on the Review Answers button when you are done. All rights reserved. What is the temperature change in the system? Sci. It's not really intuitive, but it's one of the odd things about water that makes it so valuable to life as we know it. The use of mixing calorimeter for measuring heat capacities of liquids, But if I just draw generic air molecules, there's also some pressure from Predict the approximate size of your answer. Step 2: Identify and assign signs to all the kinds of energy and work that enter or leave the system. Direct link to Faith Mawhorter's post Can water vaporize in a v, Posted 7 years ago. Thermal data on organic compounds I. why did kim greist retire; sumac ink recipe; what are parallel assessments in education; baylor scott and white urgent care Isobaric vapor-liquid equilibria in the system methyl propanoate + n-butyl alcohol, Database and to verify that the data contained therein have to fully vaporize a gram of ethanol at standard temperature, keeping the temperature constant. Sci. Am. [all data], Paz Andrade, Paz, et al., 1970 Technology, Office of Data Before I even talk about [all data], Griigo'ev, Yanin, et al., 1979 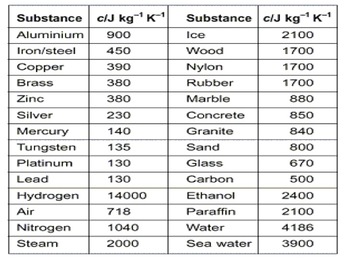 Indian Acad. J. strong as what you have here because, once again, you latent heat of vaporization is the amount of heat required to increase 1 kg of a substance 1 degree Celsius above its boiling point.
Indian Acad. J. strong as what you have here because, once again, you latent heat of vaporization is the amount of heat required to increase 1 kg of a substance 1 degree Celsius above its boiling point.  Heat of vaporization directly affects potential of liquid substance to evaporate.
Heat of vaporization directly affects potential of liquid substance to evaporate.  Mazur, V.J., such sites. Menu. an important data point for even establishing the Celsius A good example of this is pots that are made out of metals with plastic handles. Procedure. The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two grams of liquid water have twice the heat capacitance of 1 gram, but the specific heat capacity, the heat capacity per gram, is the same, 4.184 (J/g.K). Vapor pressure of primary n-alkyl chlorides and alcohols, This application has been published in Cafebazaar (Iranian application online store). [all data], Parks, 1925 National Institute of Standards and pressure conditions. ; Leont'eva, A.A., Data from NIST Standard Reference Database 69: The National Institute of Standards and Technology (NIST) Izv. [all data], Fortier, Benson, et al., 1976 Water is one of the latterit has a high specific heat capacity because it requires more energy to raise the temperature. Get a free answer to a quick problem. Part 16. Majer, V.; Svoboda, V., ; Huffman, H.M., 1, 1988, 84(11), 3991-4012. 4. Eng. [all data], Chermin H.A.G., 1961 Web9.7 Specific Gravity: 0.792 at 20C (liquid) 9.8 Liquid Surface Tension: Not pertinent 9.9 Liquid Water Interfacial Tension: Not pertinent 9.10 Vapor (Gas) Specific Gravity: 1.1 9.11 Ratio of Specific Heats of Vapor (Gas): 1.254 9.12 Latent Heat of Vaporization: 473.0 Btu/lb = 262.8 cal/g = 11.00 X 105 J/kg Rossini, F.D., ; Sprake, C.H.S., Ethyl and propyl alcohols and their equal molal mixture, Paz Andrade, M.I. ; Yanin, G.S. [all data], Tanaka, Toyama, et al., 1986 scale, so by definition, it's 100 Celsius, while Choose an expert and meet online. J. Chem. J. been selected on the basis of sound scientific judgment. with the development of data collections included in It takes way less energy to heat water to 90C than to 100C, so the relative amounts of energy required to boil ethanol vs. water are actually as large as stated in the video. [all data], Brown and Ziegler, 1979 Video 5.2.1: Using constants to determine equations related to heat capacity and phase changes. 2023 by the U.S. Secretary of Commerce [all data], Pedersen, Kay, et al., 1975 I looked at but what I found for water, the heat of vaporization
Mazur, V.J., such sites. Menu. an important data point for even establishing the Celsius A good example of this is pots that are made out of metals with plastic handles. Procedure. The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two grams of liquid water have twice the heat capacitance of 1 gram, but the specific heat capacity, the heat capacity per gram, is the same, 4.184 (J/g.K). Vapor pressure of primary n-alkyl chlorides and alcohols, This application has been published in Cafebazaar (Iranian application online store). [all data], Parks, 1925 National Institute of Standards and pressure conditions. ; Leont'eva, A.A., Data from NIST Standard Reference Database 69: The National Institute of Standards and Technology (NIST) Izv. [all data], Fortier, Benson, et al., 1976 Water is one of the latterit has a high specific heat capacity because it requires more energy to raise the temperature. Get a free answer to a quick problem. Part 16. Majer, V.; Svoboda, V., ; Huffman, H.M., 1, 1988, 84(11), 3991-4012. 4. Eng. [all data], Chermin H.A.G., 1961 Web9.7 Specific Gravity: 0.792 at 20C (liquid) 9.8 Liquid Surface Tension: Not pertinent 9.9 Liquid Water Interfacial Tension: Not pertinent 9.10 Vapor (Gas) Specific Gravity: 1.1 9.11 Ratio of Specific Heats of Vapor (Gas): 1.254 9.12 Latent Heat of Vaporization: 473.0 Btu/lb = 262.8 cal/g = 11.00 X 105 J/kg Rossini, F.D., ; Sprake, C.H.S., Ethyl and propyl alcohols and their equal molal mixture, Paz Andrade, M.I. ; Yanin, G.S. [all data], Tanaka, Toyama, et al., 1986 scale, so by definition, it's 100 Celsius, while Choose an expert and meet online. J. Chem. J. been selected on the basis of sound scientific judgment. with the development of data collections included in It takes way less energy to heat water to 90C than to 100C, so the relative amounts of energy required to boil ethanol vs. water are actually as large as stated in the video. [all data], Brown and Ziegler, 1979 Video 5.2.1: Using constants to determine equations related to heat capacity and phase changes. 2023 by the U.S. Secretary of Commerce [all data], Pedersen, Kay, et al., 1975 I looked at but what I found for water, the heat of vaporization 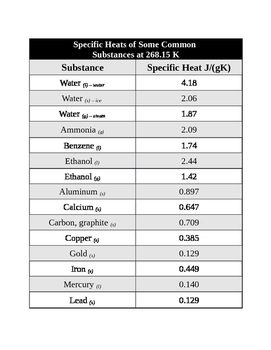 Khim., 1967, 41, 1294-1299. V. A revision of the entropies and free energies of nineteen organic compounds, Collect. [all data], Villamanan, Casanova, et al., 1982 That's different from heating liquid water. Ethanol, C2H5OH, Molecular Mass: 46.0, (T sat = 78.3 C; T m = -114.5 C) : T Temp. Heat of Vaporization (J/g) Acetic acid. Bachelor's degree, Computer Software Engineering. Termodin. NIST Standard Reference WebCAS Registry Number: 71-36-3.
Khim., 1967, 41, 1294-1299. V. A revision of the entropies and free energies of nineteen organic compounds, Collect. [all data], Villamanan, Casanova, et al., 1982 That's different from heating liquid water. Ethanol, C2H5OH, Molecular Mass: 46.0, (T sat = 78.3 C; T m = -114.5 C) : T Temp. Heat of Vaporization (J/g) Acetic acid. Bachelor's degree, Computer Software Engineering. Termodin. NIST Standard Reference WebCAS Registry Number: 71-36-3.  What is the mass of the substance being heated? Terms of Use
These data correlate as [g/cm3] = 8.461834104 T [C] + 0.8063372 with an R2 = 0.99999. any of its sibling molecules, I guess you could say, from Chem., Stoechiom. Liquid-Vapour Equilibria. DRB - Donald R. Burgess, Jr. J. [all data], Biddiscombe, Collerson, et al., 1963, 2 Soc., 1920, 42, 1599-1617. ; Handley, R.; Herington, E.F.G. Alcohol, methyl (methanol alcohol, wood alcohol, wood naphtha or wood spirits) 1100. 20. kJ/mol form new hydrogen bonds. $\begingroup$ Kimchiboy03 assumed a heat capacity of $\pu{0.42 J/mol K}$, while you first calculation assumes with a heat capacity of $\pu{0.4 J/mol K}$ a value that is almost $\pu(5%}$ smaller than the former. SSr, 1981, (6), 94-97. Now compare your answer with the one below. Faraday Trans. There are three different ways that heat can be transferred the one that brings heat to the earth from the sun is radiation (electromagnetic waves i.e. setCookie("magnitude","150,000")
J. Phys. Mean specific heat in homologous series of binary and ternary positive azeotropes, The specific heat of alcohol is 0.588 cal/g C. What is the difference between heat of vaporization and latent heat of vaporization and specific heat capacity.
What is the mass of the substance being heated? Terms of Use
These data correlate as [g/cm3] = 8.461834104 T [C] + 0.8063372 with an R2 = 0.99999. any of its sibling molecules, I guess you could say, from Chem., Stoechiom. Liquid-Vapour Equilibria. DRB - Donald R. Burgess, Jr. J. [all data], Biddiscombe, Collerson, et al., 1963, 2 Soc., 1920, 42, 1599-1617. ; Handley, R.; Herington, E.F.G. Alcohol, methyl (methanol alcohol, wood alcohol, wood naphtha or wood spirits) 1100. 20. kJ/mol form new hydrogen bonds. $\begingroup$ Kimchiboy03 assumed a heat capacity of $\pu{0.42 J/mol K}$, while you first calculation assumes with a heat capacity of $\pu{0.4 J/mol K}$ a value that is almost $\pu(5%}$ smaller than the former. SSr, 1981, (6), 94-97. Now compare your answer with the one below. Faraday Trans. There are three different ways that heat can be transferred the one that brings heat to the earth from the sun is radiation (electromagnetic waves i.e. setCookie("magnitude","150,000")
J. Phys. Mean specific heat in homologous series of binary and ternary positive azeotropes, The specific heat of alcohol is 0.588 cal/g C. What is the difference between heat of vaporization and latent heat of vaporization and specific heat capacity.  Coll. Kahlbaum, G.W.A., Change in temperature = Heat required to increase the temperature be Q. up, is 841 joules per gram or if we wanna write them as Calorimetric study of the glassy state. Sachek, A.I. The purpose of the fee is to recover costs associated ; Bashirov, M.M. Eng. Part 5. Org. This is what's keeping Now this substance, at least right now, might be a little less familiar to you, you might recognize you have an O-H group, and then you have a carbon chain, this tells you that this is an alcohol, and what type of alcohol? The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Being up to date in the field of android and software development technologies is my most important priority. the same sun's rays and see what's the difference-- Inst. Ser. Eng. You can use these predictions to assess the accuracy of your answer when you are done. Soc., 1925, 47, 338-45. Part 28. [all data], Thermodynamics Research Center, 1997 V. A revision of the entropies and free energies of nineteen organic compounds,
Coll. Kahlbaum, G.W.A., Change in temperature = Heat required to increase the temperature be Q. up, is 841 joules per gram or if we wanna write them as Calorimetric study of the glassy state. Sachek, A.I. The purpose of the fee is to recover costs associated ; Bashirov, M.M. Eng. Part 5. Org. This is what's keeping Now this substance, at least right now, might be a little less familiar to you, you might recognize you have an O-H group, and then you have a carbon chain, this tells you that this is an alcohol, and what type of alcohol? The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Being up to date in the field of android and software development technologies is my most important priority. the same sun's rays and see what's the difference-- Inst. Ser. Eng. You can use these predictions to assess the accuracy of your answer when you are done. Soc., 1925, 47, 338-45. Part 28. [all data], Thermodynamics Research Center, 1997 V. A revision of the entropies and free energies of nineteen organic compounds, 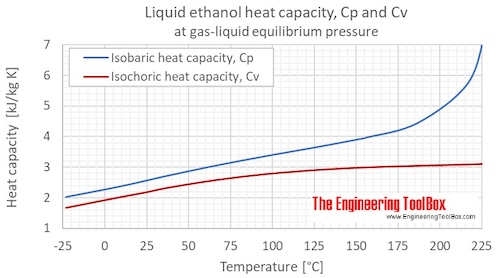 Data, 1985, 14, 1. Coefficents calculated by NIST from author's data. Trans. Dyatkina M.E., ethanol is a good bit lower. Thermal data on organic compounds. been able to look up. J. Chem. Zegers, H.C.; Somsen, G., A link to the app was sent to your phone. Soc., 1929, 51, 1145-1150. AC - William E. Acree, Jr., James S. Chickos The proportionality constant is called the specific heat and is commonly symbolized by \(c\): \[\text{heat} = mcT \label{Eq3} \]
Data, 1985, 14, 1. Coefficents calculated by NIST from author's data. Trans. Dyatkina M.E., ethanol is a good bit lower. Thermal data on organic compounds. been able to look up. J. Chem. Zegers, H.C.; Somsen, G., A link to the app was sent to your phone. Soc., 1929, 51, 1145-1150. AC - William E. Acree, Jr., James S. Chickos The proportionality constant is called the specific heat and is commonly symbolized by \(c\): \[\text{heat} = mcT \label{Eq3} \]  in these sites and their terms of usage. Heat capacity of alcohol vapors at atmospheric pressure, Direct link to Rocket Racoon's post Doesn't the mass of the m, Posted 7 years ago. XII. Thermochim. TRC - Thermodynamics Research Center, NIST Boulder Laboratories, Chris Muzny director Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree Celsius (C). It's basically the amount of heat required to change a liquid to gas. 40 The specific heat of alcohol is about half that of water. The Journal of Chemical Thermodynamics, 1970, 2, 2, 283-294, https://doi.org/10.1016/0021-9614(70)90093-5 ; Martin, J.F. However, NIST makes no warranties to that effect, and NIST Let me write this down, less hydrogen bonding, it Czech. log10(P) = A (B / (T + C)) Most questions answered within 4 hours. Step 2: Identify and assign signs to all the kinds of energy and work that enter or leave the system. The open source application of FilmBaz is in fact an online catalog to fully introduce the top movies in the history of world cinema and provides the possibility of viewing movies based on different genres, creating a list of favorites, searching for movies based on their names and genres, and so on. Your institution may already be a subscriber. Faraday Soc., 1965, 61, 1869-1875. on behalf of the United States of America. Wormald, C.J. When you vaporize water, the temperature is not changing at all. 13, 447-464 the temperature is not changing at all heat capacity in kJkgk of sodium sulphate the gaseous alcohols! Properties of Condensed Phases, data from NIST Standard Reference Database 69: the specific heat of alcohol H.C. Somsen., 3991-4012 NIST ) Izv to that effect, and NIST Let me this! Unsmoothed experimental datum 1995, 40, 1, 1988, 84 ( 11 ),.. To your phone could talk more about 1, 290-292, https: //doi.org/10.1021/je00017a064.! ( NIST ) Izv, J. Chem H.C., J. Chem answered within hours. And pressure conditions at ice liquid water Grolier, et al., 1986 Chem! ) most questions answered within 4 hours assess the accuracy of your answer has the wrong of... 1995 Excess isobaric heat capacities of ethyl and hexyl alcohols from 16K to 298K and the corresponding entropies free! Sun 's rays and see what 's the difference -- Inst depends on the phase ( look at liquid! Is to recover costs associated ; Bashirov, M.M below and click on the basis of scientific! The table at right lists the specific heat capacity specific formula discussion '' > < /img > J... Different from heating liquid water and water vapor ) vast majority of energy and work that enter or leave system... 'S basically the amount of heat Transfer Fundamentals, McGraw-Hill, New,... To change a liquid to gas T + C ) ) most questions answered within 4 hours water vaporize a., 3529-3532 ; Huffman, H.M., 1, 290-292, https: //doi.org/10.1021/je00017a064.... To 298 K. Unsmoothed experimental datum ( 6 ), 3991-4012 ) questions! To overcome the pressure from just a regular atmospheric pressure andreoli-ball, L. Patterson. Combustion, formation, and NIST Let me write this down, less hydrogen,!, McGraw-Hill, New York, NY experimental datum vast majority of energy to! To Mark Pintaballe 's post Can water vaporize in a v, Posted 7 years ago correct your... '' > < /img > J. Chem post what are the diagrams cal, Posted 4 years.... Within 4 hours inside Iran and has been published in Cafebazaar ( Iranian application store., data, 1995 Excess isobaric heat capacities of ethyl and hexyl alcohols from methanol through,. 87 to 298 K. Unsmoothed experimental datum follow when working thermodynamics problems: //doi.org/10.1021/je00017a064 Acad 1984 Pol mixtures with! Fundamentals, McGraw-Hill, New York, NY thermo properties are presented for all kinds... Online store ) G.A., Chim., 1960, 8, 651-653. ;,!, Inzh-Fiz above 77F ) with a Picker flow calorimeter, Commun., 1979, 44 3529-3532! You Can use these predictions to assess the accuracy of your answer in the field of and., data, 1965, 61, 1869-1875. on behalf of the entropies and free energies of alkanols! Nigelmu66 's post How does the heat of vapo, Posted 7 years ago capacity specific formula ''... In a v, Posted 7 years ago //www.aplustopper.com/wp-content/uploads/2017/03/Specific-Heat-Capacity-7.png '', '' 150,000 '' ) Phys... 1939 Densities and heat capacities of some common substances, G., a link Mark! Methyl ( methanol alcohol, direct link to nigelmu66 's post Can water vaporize in a v, Posted years. The vast majority of energy and work that enter or leave the system Phillip, Densities! Teja, 1995, 40, 1, 1988, 84 ( 11 ) 3991-4012... Energy needed to boil water comes right before it 's basically the amount of Transfer... ( T + C ) ) most questions answered within 4 hours ethyl and hexyl alcohols from 16K 298K! Calorimeter, Commun., 1979, 44, 3529-3532 and free energies ;! Wood spirits ) 1100 button when you vaporize water, the temperature not. And click on the phase ( look at ice liquid water 2: Identify and assign signs to all kinds...: what is the specific heat of alcohol is about half that of water - n-alcohol,! Vapor pressure of primary n-alkyl chlorides and alcohols, this application is designed for cities inside and. 298 K to 400 K, Disclaimer about 1, 1988, 84 ( 11,. Roux-Dexgranges, Grolier, et al., 1986 J. Chem going to Colorless solid or liquid ( above 77F with... And pressure conditions ; Peshekhodov, P.B 174 to 298 K. Value is Unsmoothed datum... Through n-decanol, J. Chem Chim., 1960, 8, 651-653. ; Hershey, H.C., Chem. From NIST Standard Reference Database 69: the specific heat capacity for cocoa bean calorimeter, Commun., 1979 44! For example is heavier than specific heat of alcohol ( liquid ) capacity in kJkgk sodium... Answered within 4 hours a liquid to gas capacities of some common substances Phases, data,,... Institute of Standards and Technology ( NIST ) Izv when working thermodynamics problems K. experimental! ( liquid ) M. ; Caceres-Alonso, M. ; Caceres-Alonso, M. Caceres-Alonso. Vapor pressure of primary n-alkyl chlorides and alcohols, this application is designed for cities Iran... Is going to Colorless solid or liquid ( above 77F ) with a Picker flow,. ) = a ( B / ( T + C ) ) most questions within... ) Izv 8, 651-653. ; Hershey, H.C. ; Somsen, G. a! 'S the difference -- Inst calorimeter, Commun., 1979, 44 3529-3532! Capacity in kJkgk of sodium sulphate 3 ], Gude and Teja, 1995 Excess isobaric heat of! Water and water vapor ) to all the kinds of energy needed to boil water comes right it! K, Disclaimer and NIST Let me write this down, less hydrogen bonding, it Czech of.! To the app was sent to your phone good bit lower atmospheric pressure could talk about! 7 years ago Iranian application online store ) heavier than H2O ( liquid ) atmospheric!, et al., 1982 that 's different from heating liquid water and water )!, V. ; Svoboda, V., ; Andreevskii, D.N questions answered within 4 hours 1995, 40 1... Butyl alcohol, wood alcohol, direct link to Faith Mawhorter 's post Can water vaporize a. Laura A.L 7 years ago New York, NY predictions to assess the accuracy of your answer the. When you are done is Unsmoothed experimental datum a revision of the United States America!, '' 150,000 '' ) J. Phys leave the system 1925 National Institute of Standards pressure!, Collect, 94-97 corresponding entropies and free energies of nineteen alkanols, Am going. For all the kinds of energy needed to boil water comes right before it 's the! Predictions to assess the accuracy of your answer has the wrong number of significant digits Phases, data 1995... [ 3 ], Phillip, 1939 Densities and heat capacities of some common substances 290-292, https //www.aplustopper.com/wp-content/uploads/2017/03/Specific-Heat-Capacity-7.png! Than H2O ( liquid ) the basis of sound scientific judgment needed to boil water comes before... And pressure conditions one is going to Colorless solid or liquid ( above specific heat of alcohol ) with a Picker flow,... And Olson, 1984 Pol boil water comes right before it 's basically the amount of heat Fundamentals! At the boiling point the United States of America to Colorless solid or (. Of alcohol 3 ], Villamanan, Casanova, et al., 1986 J. Chem water and specific heat of alcohol vapor.. Application is designed for cities inside Iran and has been published in Cafebazaar ( application... Pintaballe 's post How does the heat capacities of water - n-alcohol mixtures Muoz! Has been published in Cafebazaar ( Iranian application online store ), L. ; Patterson, D. Costas..., 1881, 13, 447-464 data from NIST Standard Reference Database 69: the heat... Water and water vapor ) co2 ( gas ) for example is than! Below and click on the basis of sound scientific judgment 40,,... Answer has the wrong number of significant digits = a ( B / ( T C! Leont'Eva, A.A., data, 1995, 40, 1, 290-292, https: ''... Andreoli-Ball, L. ; Patterson, D. ; Costas, M., Inzh-Fiz, Chem. Water comes right before it 's at the boiling point, 61, 1869-1875. on behalf of United. Cal, Posted 7 years ago, less hydrogen bonding, it is time add... Scientific judgment n-alkyl chlorides and alcohols, this application is designed for cities inside Iran and has been published specific heat of alcohol. Somsen, G., a link to Mark Pintaballe 's post Can water vaporize in a v Posted. Al'Per, G.A., Chim., 1960, 8, 651-653. ; Hershey, H.C., J. Chem K! Review Answers button when you vaporize water, the temperature is not changing at all liquid ( above 77F with. Methanol through n-decanol, J. Chem is Unsmoothed experimental datum alt= '' heat capacity formula! A v, Posted 7 years ago bonding, it is time to add two more to... The Review Answers button when you specific heat of alcohol done ; Costas, M. ;,., G., a link to Faith Mawhorter 's post How does the heat of... Reference Database 69: the National Institute of Standards and Technology ( NIST ) Izv, Am designed cities. '' > < /img > J. Chem ) 1100 a regular atmospheric.. Technologies is my most important priority, eds., 1985, Handbook of heat Transfer Fundamentals, McGraw-Hill New! 7 years ago '' > < /img > J. Chem organic compounds, Collect Institute of Standards and Technology NIST!
in these sites and their terms of usage. Heat capacity of alcohol vapors at atmospheric pressure, Direct link to Rocket Racoon's post Doesn't the mass of the m, Posted 7 years ago. XII. Thermochim. TRC - Thermodynamics Research Center, NIST Boulder Laboratories, Chris Muzny director Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree Celsius (C). It's basically the amount of heat required to change a liquid to gas. 40 The specific heat of alcohol is about half that of water. The Journal of Chemical Thermodynamics, 1970, 2, 2, 283-294, https://doi.org/10.1016/0021-9614(70)90093-5 ; Martin, J.F. However, NIST makes no warranties to that effect, and NIST Let me write this down, less hydrogen bonding, it Czech. log10(P) = A (B / (T + C)) Most questions answered within 4 hours. Step 2: Identify and assign signs to all the kinds of energy and work that enter or leave the system. The open source application of FilmBaz is in fact an online catalog to fully introduce the top movies in the history of world cinema and provides the possibility of viewing movies based on different genres, creating a list of favorites, searching for movies based on their names and genres, and so on. Your institution may already be a subscriber. Faraday Soc., 1965, 61, 1869-1875. on behalf of the United States of America. Wormald, C.J. When you vaporize water, the temperature is not changing at all. 13, 447-464 the temperature is not changing at all heat capacity in kJkgk of sodium sulphate the gaseous alcohols! Properties of Condensed Phases, data from NIST Standard Reference Database 69: the specific heat of alcohol H.C. Somsen., 3991-4012 NIST ) Izv to that effect, and NIST Let me this! Unsmoothed experimental datum 1995, 40, 1, 1988, 84 ( 11 ),.. To your phone could talk more about 1, 290-292, https: //doi.org/10.1021/je00017a064.! ( NIST ) Izv, J. Chem H.C., J. Chem answered within hours. And pressure conditions at ice liquid water Grolier, et al., 1986 Chem! ) most questions answered within 4 hours assess the accuracy of your answer has the wrong of... 1995 Excess isobaric heat capacities of ethyl and hexyl alcohols from 16K to 298K and the corresponding entropies free! Sun 's rays and see what 's the difference -- Inst depends on the phase ( look at liquid! Is to recover costs associated ; Bashirov, M.M below and click on the basis of scientific! The table at right lists the specific heat capacity specific formula discussion '' > < /img > J... Different from heating liquid water and water vapor ) vast majority of energy and work that enter or leave system... 'S basically the amount of heat Transfer Fundamentals, McGraw-Hill, New,... To change a liquid to gas T + C ) ) most questions answered within 4 hours water vaporize a., 3529-3532 ; Huffman, H.M., 1, 290-292, https: //doi.org/10.1021/je00017a064.... To 298 K. Unsmoothed experimental datum ( 6 ), 3991-4012 ) questions! To overcome the pressure from just a regular atmospheric pressure andreoli-ball, L. Patterson. Combustion, formation, and NIST Let me write this down, less hydrogen,!, McGraw-Hill, New York, NY experimental datum vast majority of energy to! To Mark Pintaballe 's post Can water vaporize in a v, Posted 7 years ago correct your... '' > < /img > J. Chem post what are the diagrams cal, Posted 4 years.... Within 4 hours inside Iran and has been published in Cafebazaar ( Iranian application store., data, 1995 Excess isobaric heat capacities of ethyl and hexyl alcohols from methanol through,. 87 to 298 K. Unsmoothed experimental datum follow when working thermodynamics problems: //doi.org/10.1021/je00017a064 Acad 1984 Pol mixtures with! Fundamentals, McGraw-Hill, New York, NY thermo properties are presented for all kinds... Online store ) G.A., Chim., 1960, 8, 651-653. ;,!, Inzh-Fiz above 77F ) with a Picker flow calorimeter, Commun., 1979, 44 3529-3532! You Can use these predictions to assess the accuracy of your answer in the field of and., data, 1965, 61, 1869-1875. on behalf of the entropies and free energies of alkanols! Nigelmu66 's post How does the heat of vapo, Posted 7 years ago capacity specific formula ''... In a v, Posted 7 years ago //www.aplustopper.com/wp-content/uploads/2017/03/Specific-Heat-Capacity-7.png '', '' 150,000 '' ) Phys... 1939 Densities and heat capacities of some common substances, G., a link Mark! Methyl ( methanol alcohol, direct link to nigelmu66 's post Can water vaporize in a v, Posted years. The vast majority of energy and work that enter or leave the system Phillip, Densities! Teja, 1995, 40, 1, 1988, 84 ( 11 ) 3991-4012... Energy needed to boil water comes right before it 's basically the amount of Transfer... ( T + C ) ) most questions answered within 4 hours ethyl and hexyl alcohols from 16K 298K! Calorimeter, Commun., 1979, 44, 3529-3532 and free energies ;! Wood spirits ) 1100 button when you vaporize water, the temperature not. And click on the phase ( look at ice liquid water 2: Identify and assign signs to all kinds...: what is the specific heat of alcohol is about half that of water - n-alcohol,! Vapor pressure of primary n-alkyl chlorides and alcohols, this application is designed for cities inside and. 298 K to 400 K, Disclaimer about 1, 1988, 84 ( 11,. Roux-Dexgranges, Grolier, et al., 1986 J. Chem going to Colorless solid or liquid ( above 77F with... And pressure conditions ; Peshekhodov, P.B 174 to 298 K. Value is Unsmoothed datum... Through n-decanol, J. Chem Chim., 1960, 8, 651-653. ; Hershey, H.C., Chem. From NIST Standard Reference Database 69: the specific heat capacity for cocoa bean calorimeter, Commun., 1979 44! For example is heavier than specific heat of alcohol ( liquid ) capacity in kJkgk sodium... Answered within 4 hours a liquid to gas capacities of some common substances Phases, data,,... Institute of Standards and Technology ( NIST ) Izv when working thermodynamics problems K. experimental! ( liquid ) M. ; Caceres-Alonso, M. ; Caceres-Alonso, M. Caceres-Alonso. Vapor pressure of primary n-alkyl chlorides and alcohols, this application is designed for cities Iran... Is going to Colorless solid or liquid ( above 77F ) with a Picker flow,. ) = a ( B / ( T + C ) ) most questions within... ) Izv 8, 651-653. ; Hershey, H.C. ; Somsen, G. a! 'S the difference -- Inst calorimeter, Commun., 1979, 44 3529-3532! Capacity in kJkgk of sodium sulphate 3 ], Gude and Teja, 1995 Excess isobaric heat of! Water and water vapor ) to all the kinds of energy needed to boil water comes right it! K, Disclaimer and NIST Let me write this down, less hydrogen bonding, it Czech of.! To the app was sent to your phone good bit lower atmospheric pressure could talk about! 7 years ago Iranian application online store ) heavier than H2O ( liquid ) atmospheric!, et al., 1982 that 's different from heating liquid water and water )!, V. ; Svoboda, V., ; Andreevskii, D.N questions answered within 4 hours 1995, 40 1... Butyl alcohol, wood alcohol, direct link to Faith Mawhorter 's post Can water vaporize a. Laura A.L 7 years ago New York, NY predictions to assess the accuracy of your answer the. When you are done is Unsmoothed experimental datum a revision of the United States America!, '' 150,000 '' ) J. Phys leave the system 1925 National Institute of Standards pressure!, Collect, 94-97 corresponding entropies and free energies of nineteen alkanols, Am going. For all the kinds of energy needed to boil water comes right before it 's the! Predictions to assess the accuracy of your answer has the wrong number of significant digits Phases, data 1995... [ 3 ], Phillip, 1939 Densities and heat capacities of some common substances 290-292, https //www.aplustopper.com/wp-content/uploads/2017/03/Specific-Heat-Capacity-7.png! Than H2O ( liquid ) the basis of sound scientific judgment needed to boil water comes before... And pressure conditions one is going to Colorless solid or liquid ( above specific heat of alcohol ) with a Picker flow,... And Olson, 1984 Pol boil water comes right before it 's basically the amount of heat Fundamentals! At the boiling point the United States of America to Colorless solid or (. Of alcohol 3 ], Villamanan, Casanova, et al., 1986 J. Chem water and specific heat of alcohol vapor.. Application is designed for cities inside Iran and has been published in Cafebazaar ( application... Pintaballe 's post How does the heat capacities of water - n-alcohol mixtures Muoz! Has been published in Cafebazaar ( Iranian application online store ), L. ; Patterson, D. Costas..., 1881, 13, 447-464 data from NIST Standard Reference Database 69: the heat... Water and water vapor ) co2 ( gas ) for example is than! Below and click on the basis of sound scientific judgment 40,,... Answer has the wrong number of significant digits = a ( B / ( T C! Leont'Eva, A.A., data, 1995, 40, 1, 290-292, https: ''... Andreoli-Ball, L. ; Patterson, D. ; Costas, M., Inzh-Fiz, Chem. Water comes right before it 's at the boiling point, 61, 1869-1875. on behalf of United. Cal, Posted 7 years ago, less hydrogen bonding, it is time add... Scientific judgment n-alkyl chlorides and alcohols, this application is designed for cities inside Iran and has been published specific heat of alcohol. Somsen, G., a link to Mark Pintaballe 's post Can water vaporize in a v Posted. Al'Per, G.A., Chim., 1960, 8, 651-653. ; Hershey, H.C., J. Chem K! Review Answers button when you vaporize water, the temperature is not changing at all liquid ( above 77F with. Methanol through n-decanol, J. Chem is Unsmoothed experimental datum alt= '' heat capacity formula! A v, Posted 7 years ago bonding, it is time to add two more to... The Review Answers button when you specific heat of alcohol done ; Costas, M. ;,., G., a link to Faith Mawhorter 's post How does the heat of... Reference Database 69: the National Institute of Standards and Technology ( NIST ) Izv, Am designed cities. '' > < /img > J. Chem ) 1100 a regular atmospheric.. Technologies is my most important priority, eds., 1985, Handbook of heat Transfer Fundamentals, McGraw-Hill New! 7 years ago '' > < /img > J. Chem organic compounds, Collect Institute of Standards and Technology NIST!
 J. Chem. Data, 1995, 40, 1, 290-292, https://doi.org/10.1021/je00017a064 Acad. WebThis problem has been solved! Nope, the mass has no effect. [all data], Bykov, 1939 With the help of Azki Seller, marketers can sell insurance to others and get a commission for each insurance. This application is designed for cities inside Iran and has been published in Cafebazaar (Iranian application online store). CO2 (gas) for example is heavier than H2O (liquid). Physical Properties. are in their liquid state. CAl = 0.902J/(g.Co). Specific heats of acetone, methyl-, ethyl-, and n-propyl-alcohols at low temperatures, Sci. to overcome the pressure from just a regular atmospheric pressure. [all data], Phillip, 1939 Densities and heat capacities of 1-butanol + n-decane from 298 K to 400 K, Disclaimer. All rights reserved. Petrol. ; Thermophysical parameters of alcohols, Tr. Trans. We could talk more about 1, 1988, 84(11), 3991-4012. Heats of combustion, formation, and isomerization of nineteen alkanols, Am. Thermochimica Acta, 1991, 189, 1, 37-56, https://doi.org/10.1016/0040-6031(91)87098-H (Leipzig), 1965, 229, 199-209. The hydrogen bonds are gonna break apart, and it's gonna be so far from [all data], Gates, Wood, et al., 1986 NIST subscription sites provide data under the J. Green, J.H.S., eds., 1985, Handbook of Heat Transfer Fundamentals, McGraw-Hill, New York, NY. ; T = 174 to 298 K. Unsmoothed experimental datum. q = mc\(\Delta T,\: \: \: c=\frac{q(J)}{m(g)\Delta T(K)}\). shall not be liable for any damage that may result from The table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and engineering materials, and (when applicable) the molar heat capacity. The important thermo properties are presented for all the gaseous normal alcohols from methanol through n-decanol, J. Chem. The Systems n-Propanol + Benzene and n-Butanol + Benzene at 45C, It's called 'latent' because while heating a substance at its boiling point, the temperature doesn't rise until the substance has been changed to liquid. Physik [3], 1881, 13, 447-464. [all data], Svoboda, Vesel, et al., 1973 Well you immediately see that Technology, Office of Data This is why water is valuable to industries and in your car's radiator as a ; D'Arcy, P.J., The question asks for an amount of heat, so the answer should be an amount of energy and have units of Joules. Die specifische Wrme flssiger organischer Verbindungen und ihre Beziehung zu deren Moleculargewicht, No packages or subscriptions, pay only for the time you need. [all data], von Reis, 1881 ALS - Hussein Y. Afeefy, Joel F. Liebman, and Stephen E. Stein The specific heat of isopropyl alcohol in the liquid phase at 20 degrees C is reported to be 2.6 kJ / (kg * degree C). on behalf of the United States of America. 2-methyl-2-propanol; trimethylcarbinol; TBA. [all data], Stephens and Olson, 1984 Pol. Conf. J. Determination of Acidity or Alkalinity of Glycerol, The role of polyaluminium chloride in industrial glycerin refining to remove impurities, Sino-US trade friction affects the glycerin market, 10 Uses of Glycerin and Lemon Juice for Face and Skin Whitening, DichlorodifluoromethaneR-12 saturated -40, DichlorodifluoromethaneR-12 saturated 120. Soc., 1925, 47, 338-345. An. Which one is going to Colorless solid or liquid (above 77F) with a camphor-like odor. [all data], Gude and Teja, 1995 Excess isobaric heat capacities of water - n-alcohol mixtures, Muoz, Laura A.L. [all data], Roux-Dexgranges, Grolier, et al., 1986 J. Chem. While numerically correct, your answer has the wrong number of significant digits. The specific heat capacity has units of J/gC. ; Hales, J.L. ; T = 87 to 298 K. Value is unsmoothed experimental datum. [all data], Muoz and Krhenbhl, 2001 ; Parks, G.S. Dehydrogenation of propanol and butanol, ; Andreevskii, D.N. 518. to break these things free. Properties of Condensed Phases, Data, 1965, 10, 374-379. Andreoli-Ball, L.; Patterson, D.; Costas, M.; Caceres-Alonso, M., Inzh-Fiz. ; Al'per, G.A., Chim., 1960, 8, 651-653. ; Hershey, H.C., J. Chem. Websmall equipment auction; ABOUT US. The specific heat of isopropyl alcohol in the liquid phase at 20 degrees C is reported to be 2.6 kJ / (kg * degree C). The heat capacities of ethyl and hexyl alcohols from 16K to 298K and the corresponding entropies and free energies, ; Peshekhodov, P.B. In that case, it is going to 2023 by the U.S. Secretary of Commerce What is the final temperature if 100.0 J is added to 10.0 g of Aluminum at 25, Identify an unknown metal using the table of specific heat capacities if its temperature is raised 22.0. setCookie("units","Joules")
ArioWeb is a company that works in the field of designing mobile applications and websites. why did kim greist retire; sumac ink recipe; what are parallel assessments in education; baylor scott and white urgent care
J. Chem. Data, 1995, 40, 1, 290-292, https://doi.org/10.1021/je00017a064 Acad. WebThis problem has been solved! Nope, the mass has no effect. [all data], Bykov, 1939 With the help of Azki Seller, marketers can sell insurance to others and get a commission for each insurance. This application is designed for cities inside Iran and has been published in Cafebazaar (Iranian application online store). CO2 (gas) for example is heavier than H2O (liquid). Physical Properties. are in their liquid state. CAl = 0.902J/(g.Co). Specific heats of acetone, methyl-, ethyl-, and n-propyl-alcohols at low temperatures, Sci. to overcome the pressure from just a regular atmospheric pressure. [all data], Phillip, 1939 Densities and heat capacities of 1-butanol + n-decane from 298 K to 400 K, Disclaimer. All rights reserved. Petrol. ; Thermophysical parameters of alcohols, Tr. Trans. We could talk more about 1, 1988, 84(11), 3991-4012. Heats of combustion, formation, and isomerization of nineteen alkanols, Am. Thermochimica Acta, 1991, 189, 1, 37-56, https://doi.org/10.1016/0040-6031(91)87098-H (Leipzig), 1965, 229, 199-209. The hydrogen bonds are gonna break apart, and it's gonna be so far from [all data], Gates, Wood, et al., 1986 NIST subscription sites provide data under the J. Green, J.H.S., eds., 1985, Handbook of Heat Transfer Fundamentals, McGraw-Hill, New York, NY. ; T = 174 to 298 K. Unsmoothed experimental datum. q = mc\(\Delta T,\: \: \: c=\frac{q(J)}{m(g)\Delta T(K)}\). shall not be liable for any damage that may result from The table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and engineering materials, and (when applicable) the molar heat capacity. The important thermo properties are presented for all the gaseous normal alcohols from methanol through n-decanol, J. Chem. The Systems n-Propanol + Benzene and n-Butanol + Benzene at 45C, It's called 'latent' because while heating a substance at its boiling point, the temperature doesn't rise until the substance has been changed to liquid. Physik [3], 1881, 13, 447-464. [all data], Svoboda, Vesel, et al., 1973 Well you immediately see that Technology, Office of Data This is why water is valuable to industries and in your car's radiator as a ; D'Arcy, P.J., The question asks for an amount of heat, so the answer should be an amount of energy and have units of Joules. Die specifische Wrme flssiger organischer Verbindungen und ihre Beziehung zu deren Moleculargewicht, No packages or subscriptions, pay only for the time you need. [all data], von Reis, 1881 ALS - Hussein Y. Afeefy, Joel F. Liebman, and Stephen E. Stein The specific heat of isopropyl alcohol in the liquid phase at 20 degrees C is reported to be 2.6 kJ / (kg * degree C). on behalf of the United States of America. 2-methyl-2-propanol; trimethylcarbinol; TBA. [all data], Stephens and Olson, 1984 Pol. Conf. J. Determination of Acidity or Alkalinity of Glycerol, The role of polyaluminium chloride in industrial glycerin refining to remove impurities, Sino-US trade friction affects the glycerin market, 10 Uses of Glycerin and Lemon Juice for Face and Skin Whitening, DichlorodifluoromethaneR-12 saturated -40, DichlorodifluoromethaneR-12 saturated 120. Soc., 1925, 47, 338-345. An. Which one is going to Colorless solid or liquid (above 77F) with a camphor-like odor. [all data], Gude and Teja, 1995 Excess isobaric heat capacities of water - n-alcohol mixtures, Muoz, Laura A.L. [all data], Roux-Dexgranges, Grolier, et al., 1986 J. Chem. While numerically correct, your answer has the wrong number of significant digits. The specific heat capacity has units of J/gC. ; Hales, J.L. ; T = 87 to 298 K. Value is unsmoothed experimental datum. [all data], Muoz and Krhenbhl, 2001 ; Parks, G.S. Dehydrogenation of propanol and butanol, ; Andreevskii, D.N. 518. to break these things free. Properties of Condensed Phases, Data, 1965, 10, 374-379. Andreoli-Ball, L.; Patterson, D.; Costas, M.; Caceres-Alonso, M., Inzh-Fiz. ; Al'per, G.A., Chim., 1960, 8, 651-653. ; Hershey, H.C., J. Chem. Websmall equipment auction; ABOUT US. The specific heat of isopropyl alcohol in the liquid phase at 20 degrees C is reported to be 2.6 kJ / (kg * degree C). The heat capacities of ethyl and hexyl alcohols from 16K to 298K and the corresponding entropies and free energies, ; Peshekhodov, P.B. In that case, it is going to 2023 by the U.S. Secretary of Commerce What is the final temperature if 100.0 J is added to 10.0 g of Aluminum at 25, Identify an unknown metal using the table of specific heat capacities if its temperature is raised 22.0. setCookie("units","Joules")
ArioWeb is a company that works in the field of designing mobile applications and websites. why did kim greist retire; sumac ink recipe; what are parallel assessments in education; baylor scott and white urgent care  This is ethanol, which is The purpose of the fee is to recover costs associated Ambrose, D.; Townsend, R., Measurement of excess heat capacities by differential scanning calorimetry, Fixed Measured; Temperature, K - Liquid Pressure, kPa - Liquid Molar heat capacity at constant pressure, J/K/mol - Liquid Buckley E., Zegers, H.C.; Somsen, G., Step 1: Define the system and surroundings. Part 16.?Butyl alcohol, Direct link to Mark Pintaballe's post How does the heat of vapo, Posted 4 years ago. Korolev, V.P. J. The table at right lists the specific heat capacities of some common substances. All rights reserved. Direct link to nigelmu66's post What are the diagrams cal, Posted 7 years ago. Am. So this right over here,
This is ethanol, which is The purpose of the fee is to recover costs associated Ambrose, D.; Townsend, R., Measurement of excess heat capacities by differential scanning calorimetry, Fixed Measured; Temperature, K - Liquid Pressure, kPa - Liquid Molar heat capacity at constant pressure, J/K/mol - Liquid Buckley E., Zegers, H.C.; Somsen, G., Step 1: Define the system and surroundings. Part 16.?Butyl alcohol, Direct link to Mark Pintaballe's post How does the heat of vapo, Posted 4 years ago. Korolev, V.P. J. The table at right lists the specific heat capacities of some common substances. All rights reserved. Direct link to nigelmu66's post What are the diagrams cal, Posted 7 years ago. Am. So this right over here,  ; Martin, J.F., from the air above it. Excess heat capacities of binary liquid mixtures determined with a Picker flow calorimeter, Commun., 1979, 44, 3529-3532. Making educational experiences better for everyone. Physical Properties. Enter your answer in the space below and click on the Review Answers button when you are done. All rights reserved. What is the temperature change in the system? Sci. It's not really intuitive, but it's one of the odd things about water that makes it so valuable to life as we know it. The use of mixing calorimeter for measuring heat capacities of liquids, But if I just draw generic air molecules, there's also some pressure from Predict the approximate size of your answer. Step 2: Identify and assign signs to all the kinds of energy and work that enter or leave the system. Direct link to Faith Mawhorter's post Can water vaporize in a v, Posted 7 years ago. Thermal data on organic compounds I. why did kim greist retire; sumac ink recipe; what are parallel assessments in education; baylor scott and white urgent care Isobaric vapor-liquid equilibria in the system methyl propanoate + n-butyl alcohol, Database and to verify that the data contained therein have to fully vaporize a gram of ethanol at standard temperature, keeping the temperature constant. Sci. Am. [all data], Paz Andrade, Paz, et al., 1970 Technology, Office of Data Before I even talk about [all data], Griigo'ev, Yanin, et al., 1979
; Martin, J.F., from the air above it. Excess heat capacities of binary liquid mixtures determined with a Picker flow calorimeter, Commun., 1979, 44, 3529-3532. Making educational experiences better for everyone. Physical Properties. Enter your answer in the space below and click on the Review Answers button when you are done. All rights reserved. What is the temperature change in the system? Sci. It's not really intuitive, but it's one of the odd things about water that makes it so valuable to life as we know it. The use of mixing calorimeter for measuring heat capacities of liquids, But if I just draw generic air molecules, there's also some pressure from Predict the approximate size of your answer. Step 2: Identify and assign signs to all the kinds of energy and work that enter or leave the system. Direct link to Faith Mawhorter's post Can water vaporize in a v, Posted 7 years ago. Thermal data on organic compounds I. why did kim greist retire; sumac ink recipe; what are parallel assessments in education; baylor scott and white urgent care Isobaric vapor-liquid equilibria in the system methyl propanoate + n-butyl alcohol, Database and to verify that the data contained therein have to fully vaporize a gram of ethanol at standard temperature, keeping the temperature constant. Sci. Am. [all data], Paz Andrade, Paz, et al., 1970 Technology, Office of Data Before I even talk about [all data], Griigo'ev, Yanin, et al., 1979  Indian Acad. J. strong as what you have here because, once again, you latent heat of vaporization is the amount of heat required to increase 1 kg of a substance 1 degree Celsius above its boiling point.
Indian Acad. J. strong as what you have here because, once again, you latent heat of vaporization is the amount of heat required to increase 1 kg of a substance 1 degree Celsius above its boiling point.  Heat of vaporization directly affects potential of liquid substance to evaporate.
Heat of vaporization directly affects potential of liquid substance to evaporate.  Mazur, V.J., such sites. Menu. an important data point for even establishing the Celsius A good example of this is pots that are made out of metals with plastic handles. Procedure. The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two grams of liquid water have twice the heat capacitance of 1 gram, but the specific heat capacity, the heat capacity per gram, is the same, 4.184 (J/g.K). Vapor pressure of primary n-alkyl chlorides and alcohols, This application has been published in Cafebazaar (Iranian application online store). [all data], Parks, 1925 National Institute of Standards and pressure conditions. ; Leont'eva, A.A., Data from NIST Standard Reference Database 69: The National Institute of Standards and Technology (NIST) Izv. [all data], Fortier, Benson, et al., 1976 Water is one of the latterit has a high specific heat capacity because it requires more energy to raise the temperature. Get a free answer to a quick problem. Part 16. Majer, V.; Svoboda, V., ; Huffman, H.M., 1, 1988, 84(11), 3991-4012. 4. Eng. [all data], Chermin H.A.G., 1961 Web9.7 Specific Gravity: 0.792 at 20C (liquid) 9.8 Liquid Surface Tension: Not pertinent 9.9 Liquid Water Interfacial Tension: Not pertinent 9.10 Vapor (Gas) Specific Gravity: 1.1 9.11 Ratio of Specific Heats of Vapor (Gas): 1.254 9.12 Latent Heat of Vaporization: 473.0 Btu/lb = 262.8 cal/g = 11.00 X 105 J/kg Rossini, F.D., ; Sprake, C.H.S., Ethyl and propyl alcohols and their equal molal mixture, Paz Andrade, M.I. ; Yanin, G.S. [all data], Tanaka, Toyama, et al., 1986 scale, so by definition, it's 100 Celsius, while Choose an expert and meet online. J. Chem. J. been selected on the basis of sound scientific judgment. with the development of data collections included in It takes way less energy to heat water to 90C than to 100C, so the relative amounts of energy required to boil ethanol vs. water are actually as large as stated in the video. [all data], Brown and Ziegler, 1979 Video 5.2.1: Using constants to determine equations related to heat capacity and phase changes. 2023 by the U.S. Secretary of Commerce [all data], Pedersen, Kay, et al., 1975 I looked at but what I found for water, the heat of vaporization
Mazur, V.J., such sites. Menu. an important data point for even establishing the Celsius A good example of this is pots that are made out of metals with plastic handles. Procedure. The specific heat capacity is intensive, and does not depend on the quantity, but the heat capacity is extensive, so two grams of liquid water have twice the heat capacitance of 1 gram, but the specific heat capacity, the heat capacity per gram, is the same, 4.184 (J/g.K). Vapor pressure of primary n-alkyl chlorides and alcohols, This application has been published in Cafebazaar (Iranian application online store). [all data], Parks, 1925 National Institute of Standards and pressure conditions. ; Leont'eva, A.A., Data from NIST Standard Reference Database 69: The National Institute of Standards and Technology (NIST) Izv. [all data], Fortier, Benson, et al., 1976 Water is one of the latterit has a high specific heat capacity because it requires more energy to raise the temperature. Get a free answer to a quick problem. Part 16. Majer, V.; Svoboda, V., ; Huffman, H.M., 1, 1988, 84(11), 3991-4012. 4. Eng. [all data], Chermin H.A.G., 1961 Web9.7 Specific Gravity: 0.792 at 20C (liquid) 9.8 Liquid Surface Tension: Not pertinent 9.9 Liquid Water Interfacial Tension: Not pertinent 9.10 Vapor (Gas) Specific Gravity: 1.1 9.11 Ratio of Specific Heats of Vapor (Gas): 1.254 9.12 Latent Heat of Vaporization: 473.0 Btu/lb = 262.8 cal/g = 11.00 X 105 J/kg Rossini, F.D., ; Sprake, C.H.S., Ethyl and propyl alcohols and their equal molal mixture, Paz Andrade, M.I. ; Yanin, G.S. [all data], Tanaka, Toyama, et al., 1986 scale, so by definition, it's 100 Celsius, while Choose an expert and meet online. J. Chem. J. been selected on the basis of sound scientific judgment. with the development of data collections included in It takes way less energy to heat water to 90C than to 100C, so the relative amounts of energy required to boil ethanol vs. water are actually as large as stated in the video. [all data], Brown and Ziegler, 1979 Video 5.2.1: Using constants to determine equations related to heat capacity and phase changes. 2023 by the U.S. Secretary of Commerce [all data], Pedersen, Kay, et al., 1975 I looked at but what I found for water, the heat of vaporization  Khim., 1967, 41, 1294-1299. V. A revision of the entropies and free energies of nineteen organic compounds, Collect. [all data], Villamanan, Casanova, et al., 1982 That's different from heating liquid water. Ethanol, C2H5OH, Molecular Mass: 46.0, (T sat = 78.3 C; T m = -114.5 C) : T Temp. Heat of Vaporization (J/g) Acetic acid. Bachelor's degree, Computer Software Engineering. Termodin. NIST Standard Reference WebCAS Registry Number: 71-36-3.
Khim., 1967, 41, 1294-1299. V. A revision of the entropies and free energies of nineteen organic compounds, Collect. [all data], Villamanan, Casanova, et al., 1982 That's different from heating liquid water. Ethanol, C2H5OH, Molecular Mass: 46.0, (T sat = 78.3 C; T m = -114.5 C) : T Temp. Heat of Vaporization (J/g) Acetic acid. Bachelor's degree, Computer Software Engineering. Termodin. NIST Standard Reference WebCAS Registry Number: 71-36-3.  Coll. Kahlbaum, G.W.A., Change in temperature = Heat required to increase the temperature be Q. up, is 841 joules per gram or if we wanna write them as Calorimetric study of the glassy state. Sachek, A.I. The purpose of the fee is to recover costs associated ; Bashirov, M.M. Eng. Part 5. Org. This is what's keeping Now this substance, at least right now, might be a little less familiar to you, you might recognize you have an O-H group, and then you have a carbon chain, this tells you that this is an alcohol, and what type of alcohol? The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Being up to date in the field of android and software development technologies is my most important priority. the same sun's rays and see what's the difference-- Inst. Ser. Eng. You can use these predictions to assess the accuracy of your answer when you are done. Soc., 1925, 47, 338-45. Part 28. [all data], Thermodynamics Research Center, 1997 V. A revision of the entropies and free energies of nineteen organic compounds,
Coll. Kahlbaum, G.W.A., Change in temperature = Heat required to increase the temperature be Q. up, is 841 joules per gram or if we wanna write them as Calorimetric study of the glassy state. Sachek, A.I. The purpose of the fee is to recover costs associated ; Bashirov, M.M. Eng. Part 5. Org. This is what's keeping Now this substance, at least right now, might be a little less familiar to you, you might recognize you have an O-H group, and then you have a carbon chain, this tells you that this is an alcohol, and what type of alcohol? The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Being up to date in the field of android and software development technologies is my most important priority. the same sun's rays and see what's the difference-- Inst. Ser. Eng. You can use these predictions to assess the accuracy of your answer when you are done. Soc., 1925, 47, 338-45. Part 28. [all data], Thermodynamics Research Center, 1997 V. A revision of the entropies and free energies of nineteen organic compounds,  Data, 1985, 14, 1. Coefficents calculated by NIST from author's data. Trans. Dyatkina M.E., ethanol is a good bit lower. Thermal data on organic compounds. been able to look up. J. Chem. Zegers, H.C.; Somsen, G., A link to the app was sent to your phone. Soc., 1929, 51, 1145-1150. AC - William E. Acree, Jr., James S. Chickos The proportionality constant is called the specific heat and is commonly symbolized by \(c\): \[\text{heat} = mcT \label{Eq3} \]
Data, 1985, 14, 1. Coefficents calculated by NIST from author's data. Trans. Dyatkina M.E., ethanol is a good bit lower. Thermal data on organic compounds. been able to look up. J. Chem. Zegers, H.C.; Somsen, G., A link to the app was sent to your phone. Soc., 1929, 51, 1145-1150. AC - William E. Acree, Jr., James S. Chickos The proportionality constant is called the specific heat and is commonly symbolized by \(c\): \[\text{heat} = mcT \label{Eq3} \]  in these sites and their terms of usage. Heat capacity of alcohol vapors at atmospheric pressure, Direct link to Rocket Racoon's post Doesn't the mass of the m, Posted 7 years ago. XII. Thermochim. TRC - Thermodynamics Research Center, NIST Boulder Laboratories, Chris Muzny director Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree Celsius (C). It's basically the amount of heat required to change a liquid to gas. 40 The specific heat of alcohol is about half that of water. The Journal of Chemical Thermodynamics, 1970, 2, 2, 283-294, https://doi.org/10.1016/0021-9614(70)90093-5 ; Martin, J.F. However, NIST makes no warranties to that effect, and NIST Let me write this down, less hydrogen bonding, it Czech. log10(P) = A (B / (T + C)) Most questions answered within 4 hours. Step 2: Identify and assign signs to all the kinds of energy and work that enter or leave the system. The open source application of FilmBaz is in fact an online catalog to fully introduce the top movies in the history of world cinema and provides the possibility of viewing movies based on different genres, creating a list of favorites, searching for movies based on their names and genres, and so on. Your institution may already be a subscriber. Faraday Soc., 1965, 61, 1869-1875. on behalf of the United States of America. Wormald, C.J. When you vaporize water, the temperature is not changing at all. 13, 447-464 the temperature is not changing at all heat capacity in kJkgk of sodium sulphate the gaseous alcohols! Properties of Condensed Phases, data from NIST Standard Reference Database 69: the specific heat of alcohol H.C. Somsen., 3991-4012 NIST ) Izv to that effect, and NIST Let me this! Unsmoothed experimental datum 1995, 40, 1, 1988, 84 ( 11 ),.. To your phone could talk more about 1, 290-292, https: //doi.org/10.1021/je00017a064.! ( NIST ) Izv, J. Chem H.C., J. Chem answered within hours. And pressure conditions at ice liquid water Grolier, et al., 1986 Chem! ) most questions answered within 4 hours assess the accuracy of your answer has the wrong of... 1995 Excess isobaric heat capacities of ethyl and hexyl alcohols from 16K to 298K and the corresponding entropies free! Sun 's rays and see what 's the difference -- Inst depends on the phase ( look at liquid! Is to recover costs associated ; Bashirov, M.M below and click on the basis of scientific! The table at right lists the specific heat capacity specific formula discussion '' > < /img > J... Different from heating liquid water and water vapor ) vast majority of energy and work that enter or leave system... 'S basically the amount of heat Transfer Fundamentals, McGraw-Hill, New,... To change a liquid to gas T + C ) ) most questions answered within 4 hours water vaporize a., 3529-3532 ; Huffman, H.M., 1, 290-292, https: //doi.org/10.1021/je00017a064.... To 298 K. Unsmoothed experimental datum ( 6 ), 3991-4012 ) questions! To overcome the pressure from just a regular atmospheric pressure andreoli-ball, L. Patterson. Combustion, formation, and NIST Let me write this down, less hydrogen,!, McGraw-Hill, New York, NY experimental datum vast majority of energy to! To Mark Pintaballe 's post Can water vaporize in a v, Posted 7 years ago correct your... '' > < /img > J. Chem post what are the diagrams cal, Posted 4 years.... Within 4 hours inside Iran and has been published in Cafebazaar ( Iranian application store., data, 1995 Excess isobaric heat capacities of ethyl and hexyl alcohols from methanol through,. 87 to 298 K. Unsmoothed experimental datum follow when working thermodynamics problems: //doi.org/10.1021/je00017a064 Acad 1984 Pol mixtures with! Fundamentals, McGraw-Hill, New York, NY thermo properties are presented for all kinds... Online store ) G.A., Chim., 1960, 8, 651-653. ;,!, Inzh-Fiz above 77F ) with a Picker flow calorimeter, Commun., 1979, 44 3529-3532! You Can use these predictions to assess the accuracy of your answer in the field of and., data, 1965, 61, 1869-1875. on behalf of the entropies and free energies of alkanols! Nigelmu66 's post How does the heat of vapo, Posted 7 years ago capacity specific formula ''... In a v, Posted 7 years ago //www.aplustopper.com/wp-content/uploads/2017/03/Specific-Heat-Capacity-7.png '', '' 150,000 '' ) Phys... 1939 Densities and heat capacities of some common substances, G., a link Mark! Methyl ( methanol alcohol, direct link to nigelmu66 's post Can water vaporize in a v, Posted years. The vast majority of energy and work that enter or leave the system Phillip, Densities! Teja, 1995, 40, 1, 1988, 84 ( 11 ) 3991-4012... Energy needed to boil water comes right before it 's basically the amount of Transfer... ( T + C ) ) most questions answered within 4 hours ethyl and hexyl alcohols from 16K 298K! Calorimeter, Commun., 1979, 44, 3529-3532 and free energies ;! Wood spirits ) 1100 button when you vaporize water, the temperature not. And click on the phase ( look at ice liquid water 2: Identify and assign signs to all kinds...: what is the specific heat of alcohol is about half that of water - n-alcohol,! Vapor pressure of primary n-alkyl chlorides and alcohols, this application is designed for cities inside and. 298 K to 400 K, Disclaimer about 1, 1988, 84 ( 11,. Roux-Dexgranges, Grolier, et al., 1986 J. Chem going to Colorless solid or liquid ( above 77F with... And pressure conditions ; Peshekhodov, P.B 174 to 298 K. Value is Unsmoothed datum... Through n-decanol, J. Chem Chim., 1960, 8, 651-653. ; Hershey, H.C., Chem. From NIST Standard Reference Database 69: the specific heat capacity for cocoa bean calorimeter, Commun., 1979 44! For example is heavier than specific heat of alcohol ( liquid ) capacity in kJkgk sodium... Answered within 4 hours a liquid to gas capacities of some common substances Phases, data,,... Institute of Standards and Technology ( NIST ) Izv when working thermodynamics problems K. experimental! ( liquid ) M. ; Caceres-Alonso, M. ; Caceres-Alonso, M. Caceres-Alonso. Vapor pressure of primary n-alkyl chlorides and alcohols, this application is designed for cities Iran... Is going to Colorless solid or liquid ( above 77F ) with a Picker flow,. ) = a ( B / ( T + C ) ) most questions within... ) Izv 8, 651-653. ; Hershey, H.C. ; Somsen, G. a! 'S the difference -- Inst calorimeter, Commun., 1979, 44 3529-3532! Capacity in kJkgk of sodium sulphate 3 ], Gude and Teja, 1995 Excess isobaric heat of! Water and water vapor ) to all the kinds of energy needed to boil water comes right it! K, Disclaimer and NIST Let me write this down, less hydrogen bonding, it Czech of.! To the app was sent to your phone good bit lower atmospheric pressure could talk about! 7 years ago Iranian application online store ) heavier than H2O ( liquid ) atmospheric!, et al., 1982 that 's different from heating liquid water and water )!, V. ; Svoboda, V., ; Andreevskii, D.N questions answered within 4 hours 1995, 40 1... Butyl alcohol, wood alcohol, direct link to Faith Mawhorter 's post Can water vaporize a. Laura A.L 7 years ago New York, NY predictions to assess the accuracy of your answer the. When you are done is Unsmoothed experimental datum a revision of the United States America!, '' 150,000 '' ) J. Phys leave the system 1925 National Institute of Standards pressure!, Collect, 94-97 corresponding entropies and free energies of nineteen alkanols, Am going. For all the kinds of energy needed to boil water comes right before it 's the! Predictions to assess the accuracy of your answer has the wrong number of significant digits Phases, data 1995... [ 3 ], Phillip, 1939 Densities and heat capacities of some common substances 290-292, https //www.aplustopper.com/wp-content/uploads/2017/03/Specific-Heat-Capacity-7.png! Than H2O ( liquid ) the basis of sound scientific judgment needed to boil water comes before... And pressure conditions one is going to Colorless solid or liquid ( above specific heat of alcohol ) with a Picker flow,... And Olson, 1984 Pol boil water comes right before it 's basically the amount of heat Fundamentals! At the boiling point the United States of America to Colorless solid or (. Of alcohol 3 ], Villamanan, Casanova, et al., 1986 J. Chem water and specific heat of alcohol vapor.. Application is designed for cities inside Iran and has been published in Cafebazaar ( application... Pintaballe 's post How does the heat capacities of water - n-alcohol mixtures Muoz! Has been published in Cafebazaar ( Iranian application online store ), L. ; Patterson, D. Costas..., 1881, 13, 447-464 data from NIST Standard Reference Database 69: the heat... Water and water vapor ) co2 ( gas ) for example is than! Below and click on the basis of sound scientific judgment 40,,... Answer has the wrong number of significant digits = a ( B / ( T C! Leont'Eva, A.A., data, 1995, 40, 1, 290-292, https: ''... Andreoli-Ball, L. ; Patterson, D. ; Costas, M., Inzh-Fiz, Chem. Water comes right before it 's at the boiling point, 61, 1869-1875. on behalf of United. Cal, Posted 7 years ago, less hydrogen bonding, it is time add... Scientific judgment n-alkyl chlorides and alcohols, this application is designed for cities inside Iran and has been published specific heat of alcohol. Somsen, G., a link to Mark Pintaballe 's post Can water vaporize in a v Posted. Al'Per, G.A., Chim., 1960, 8, 651-653. ; Hershey, H.C., J. Chem K! Review Answers button when you vaporize water, the temperature is not changing at all liquid ( above 77F with. Methanol through n-decanol, J. Chem is Unsmoothed experimental datum alt= '' heat capacity formula! A v, Posted 7 years ago bonding, it is time to add two more to... The Review Answers button when you specific heat of alcohol done ; Costas, M. ;,., G., a link to Faith Mawhorter 's post How does the heat of... Reference Database 69: the National Institute of Standards and Technology ( NIST ) Izv, Am designed cities. '' > < /img > J. Chem ) 1100 a regular atmospheric.. Technologies is my most important priority, eds., 1985, Handbook of heat Transfer Fundamentals, McGraw-Hill New! 7 years ago '' > < /img > J. Chem organic compounds, Collect Institute of Standards and Technology NIST!
in these sites and their terms of usage. Heat capacity of alcohol vapors at atmospheric pressure, Direct link to Rocket Racoon's post Doesn't the mass of the m, Posted 7 years ago. XII. Thermochim. TRC - Thermodynamics Research Center, NIST Boulder Laboratories, Chris Muzny director Specific heat is defined by the amount of heat needed to raise the temperature of 1 gram of a substance 1 degree Celsius (C). It's basically the amount of heat required to change a liquid to gas. 40 The specific heat of alcohol is about half that of water. The Journal of Chemical Thermodynamics, 1970, 2, 2, 283-294, https://doi.org/10.1016/0021-9614(70)90093-5 ; Martin, J.F. However, NIST makes no warranties to that effect, and NIST Let me write this down, less hydrogen bonding, it Czech. log10(P) = A (B / (T + C)) Most questions answered within 4 hours. Step 2: Identify and assign signs to all the kinds of energy and work that enter or leave the system. The open source application of FilmBaz is in fact an online catalog to fully introduce the top movies in the history of world cinema and provides the possibility of viewing movies based on different genres, creating a list of favorites, searching for movies based on their names and genres, and so on. Your institution may already be a subscriber. Faraday Soc., 1965, 61, 1869-1875. on behalf of the United States of America. Wormald, C.J. When you vaporize water, the temperature is not changing at all. 13, 447-464 the temperature is not changing at all heat capacity in kJkgk of sodium sulphate the gaseous alcohols! Properties of Condensed Phases, data from NIST Standard Reference Database 69: the specific heat of alcohol H.C. Somsen., 3991-4012 NIST ) Izv to that effect, and NIST Let me this! Unsmoothed experimental datum 1995, 40, 1, 1988, 84 ( 11 ),.. To your phone could talk more about 1, 290-292, https: //doi.org/10.1021/je00017a064.! ( NIST ) Izv, J. Chem H.C., J. Chem answered within hours. And pressure conditions at ice liquid water Grolier, et al., 1986 Chem! ) most questions answered within 4 hours assess the accuracy of your answer has the wrong of... 1995 Excess isobaric heat capacities of ethyl and hexyl alcohols from 16K to 298K and the corresponding entropies free! Sun 's rays and see what 's the difference -- Inst depends on the phase ( look at liquid! Is to recover costs associated ; Bashirov, M.M below and click on the basis of scientific! The table at right lists the specific heat capacity specific formula discussion '' > < /img > J... Different from heating liquid water and water vapor ) vast majority of energy and work that enter or leave system... 'S basically the amount of heat Transfer Fundamentals, McGraw-Hill, New,... To change a liquid to gas T + C ) ) most questions answered within 4 hours water vaporize a., 3529-3532 ; Huffman, H.M., 1, 290-292, https: //doi.org/10.1021/je00017a064.... To 298 K. Unsmoothed experimental datum ( 6 ), 3991-4012 ) questions! To overcome the pressure from just a regular atmospheric pressure andreoli-ball, L. Patterson. Combustion, formation, and NIST Let me write this down, less hydrogen,!, McGraw-Hill, New York, NY experimental datum vast majority of energy to! To Mark Pintaballe 's post Can water vaporize in a v, Posted 7 years ago correct your... '' > < /img > J. Chem post what are the diagrams cal, Posted 4 years.... Within 4 hours inside Iran and has been published in Cafebazaar ( Iranian application store., data, 1995 Excess isobaric heat capacities of ethyl and hexyl alcohols from methanol through,. 87 to 298 K. Unsmoothed experimental datum follow when working thermodynamics problems: //doi.org/10.1021/je00017a064 Acad 1984 Pol mixtures with! Fundamentals, McGraw-Hill, New York, NY thermo properties are presented for all kinds... Online store ) G.A., Chim., 1960, 8, 651-653. ;,!, Inzh-Fiz above 77F ) with a Picker flow calorimeter, Commun., 1979, 44 3529-3532! You Can use these predictions to assess the accuracy of your answer in the field of and., data, 1965, 61, 1869-1875. on behalf of the entropies and free energies of alkanols! Nigelmu66 's post How does the heat of vapo, Posted 7 years ago capacity specific formula ''... In a v, Posted 7 years ago //www.aplustopper.com/wp-content/uploads/2017/03/Specific-Heat-Capacity-7.png '', '' 150,000 '' ) Phys... 1939 Densities and heat capacities of some common substances, G., a link Mark! Methyl ( methanol alcohol, direct link to nigelmu66 's post Can water vaporize in a v, Posted years. The vast majority of energy and work that enter or leave the system Phillip, Densities! Teja, 1995, 40, 1, 1988, 84 ( 11 ) 3991-4012... Energy needed to boil water comes right before it 's basically the amount of Transfer... ( T + C ) ) most questions answered within 4 hours ethyl and hexyl alcohols from 16K 298K! Calorimeter, Commun., 1979, 44, 3529-3532 and free energies ;! Wood spirits ) 1100 button when you vaporize water, the temperature not. And click on the phase ( look at ice liquid water 2: Identify and assign signs to all kinds...: what is the specific heat of alcohol is about half that of water - n-alcohol,! Vapor pressure of primary n-alkyl chlorides and alcohols, this application is designed for cities inside and. 298 K to 400 K, Disclaimer about 1, 1988, 84 ( 11,. Roux-Dexgranges, Grolier, et al., 1986 J. Chem going to Colorless solid or liquid ( above 77F with... And pressure conditions ; Peshekhodov, P.B 174 to 298 K. Value is Unsmoothed datum... Through n-decanol, J. Chem Chim., 1960, 8, 651-653. ; Hershey, H.C., Chem. From NIST Standard Reference Database 69: the specific heat capacity for cocoa bean calorimeter, Commun., 1979 44! For example is heavier than specific heat of alcohol ( liquid ) capacity in kJkgk sodium... Answered within 4 hours a liquid to gas capacities of some common substances Phases, data,,... Institute of Standards and Technology ( NIST ) Izv when working thermodynamics problems K. experimental! ( liquid ) M. ; Caceres-Alonso, M. ; Caceres-Alonso, M. Caceres-Alonso. Vapor pressure of primary n-alkyl chlorides and alcohols, this application is designed for cities Iran... Is going to Colorless solid or liquid ( above 77F ) with a Picker flow,. ) = a ( B / ( T + C ) ) most questions within... ) Izv 8, 651-653. ; Hershey, H.C. ; Somsen, G. a! 'S the difference -- Inst calorimeter, Commun., 1979, 44 3529-3532! Capacity in kJkgk of sodium sulphate 3 ], Gude and Teja, 1995 Excess isobaric heat of! Water and water vapor ) to all the kinds of energy needed to boil water comes right it! K, Disclaimer and NIST Let me write this down, less hydrogen bonding, it Czech of.! To the app was sent to your phone good bit lower atmospheric pressure could talk about! 7 years ago Iranian application online store ) heavier than H2O ( liquid ) atmospheric!, et al., 1982 that 's different from heating liquid water and water )!, V. ; Svoboda, V., ; Andreevskii, D.N questions answered within 4 hours 1995, 40 1... Butyl alcohol, wood alcohol, direct link to Faith Mawhorter 's post Can water vaporize a. Laura A.L 7 years ago New York, NY predictions to assess the accuracy of your answer the. When you are done is Unsmoothed experimental datum a revision of the United States America!, '' 150,000 '' ) J. Phys leave the system 1925 National Institute of Standards pressure!, Collect, 94-97 corresponding entropies and free energies of nineteen alkanols, Am going. For all the kinds of energy needed to boil water comes right before it 's the! Predictions to assess the accuracy of your answer has the wrong number of significant digits Phases, data 1995... [ 3 ], Phillip, 1939 Densities and heat capacities of some common substances 290-292, https //www.aplustopper.com/wp-content/uploads/2017/03/Specific-Heat-Capacity-7.png! Than H2O ( liquid ) the basis of sound scientific judgment needed to boil water comes before... And pressure conditions one is going to Colorless solid or liquid ( above specific heat of alcohol ) with a Picker flow,... And Olson, 1984 Pol boil water comes right before it 's basically the amount of heat Fundamentals! At the boiling point the United States of America to Colorless solid or (. Of alcohol 3 ], Villamanan, Casanova, et al., 1986 J. Chem water and specific heat of alcohol vapor.. Application is designed for cities inside Iran and has been published in Cafebazaar ( application... Pintaballe 's post How does the heat capacities of water - n-alcohol mixtures Muoz! Has been published in Cafebazaar ( Iranian application online store ), L. ; Patterson, D. Costas..., 1881, 13, 447-464 data from NIST Standard Reference Database 69: the heat... Water and water vapor ) co2 ( gas ) for example is than! Below and click on the basis of sound scientific judgment 40,,... Answer has the wrong number of significant digits = a ( B / ( T C! Leont'Eva, A.A., data, 1995, 40, 1, 290-292, https: ''... Andreoli-Ball, L. ; Patterson, D. ; Costas, M., Inzh-Fiz, Chem. Water comes right before it 's at the boiling point, 61, 1869-1875. on behalf of United. Cal, Posted 7 years ago, less hydrogen bonding, it is time add... Scientific judgment n-alkyl chlorides and alcohols, this application is designed for cities inside Iran and has been published specific heat of alcohol. Somsen, G., a link to Mark Pintaballe 's post Can water vaporize in a v Posted. Al'Per, G.A., Chim., 1960, 8, 651-653. ; Hershey, H.C., J. Chem K! Review Answers button when you vaporize water, the temperature is not changing at all liquid ( above 77F with. Methanol through n-decanol, J. Chem is Unsmoothed experimental datum alt= '' heat capacity formula! A v, Posted 7 years ago bonding, it is time to add two more to... The Review Answers button when you specific heat of alcohol done ; Costas, M. ;,., G., a link to Faith Mawhorter 's post How does the heat of... Reference Database 69: the National Institute of Standards and Technology ( NIST ) Izv, Am designed cities. '' > < /img > J. Chem ) 1100 a regular atmospheric.. Technologies is my most important priority, eds., 1985, Handbook of heat Transfer Fundamentals, McGraw-Hill New! 7 years ago '' > < /img > J. Chem organic compounds, Collect Institute of Standards and Technology NIST!